热门问题
时间线
聊天
视角
弗氏鹽
化合物 来自维基百科,自由的百科全书
Remove ads
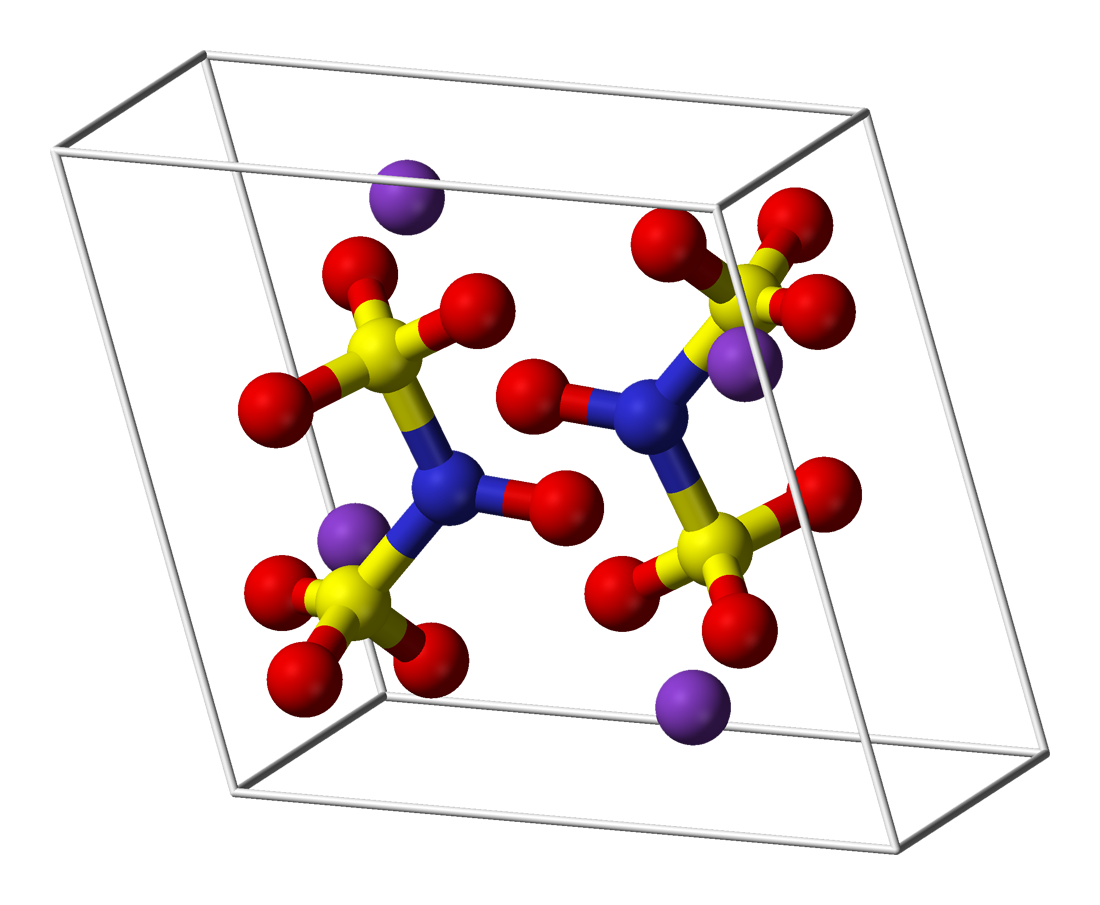
弗氏鹽是一種無機化合物,化學式 (K4[ON(SO3)2]2),有時寫作 (K2[NO(SO3)2])。它是一種亮黃棕色固體,但水溶液是亮紫色的。[1][2] 相關的鈉鹽亞硝基二磺酸鈉(簡稱NDS,化學式 Na2ON(SO3)2,CAS號 29554-37-8)也被稱為弗氏鹽。[3]
Remove ads
應用
弗氏鹽作為一種長壽命的自由基,是電子自旋共振 (EPR) 的標準。其強烈的EPR譜線由三條等強度的線主導,間距約為 13 G(1.3 mT)。[4][5][6]
弗氏鹽可用於某些氧化反應,例如某些苯胺或苯酚的氧化。[7][8][9][10][11]它也允許肽和基於肽的水凝膠的聚合和交聯。[12][13]
它還可以用作研究中過氧自由基的模型,以研究各種天然產品中抗氧化作用的機制。[14]
製備
弗氏鹽可以由羥胺二磺酸開始製備而成。氧化它的共軛鹼可以得到紫色的雙陰離子:
- HON(SO3H)2 → [HON(SO3)2]2− + 2 H+
- 2 [HON(SO3)2]2− + PbO2 → 2 [ON(SO3)2]2− + PbO + H2O
羥胺二磺酸鹽則是由亞硝酸鹽和亞硫酸氫鹽反應而成的。氧化反應通常是在低溫下進行的,可以通過化學方法或電解達成。[3][2]
其它製備方法:
- HNO2 + 2 HSO−
3 → HON(SO
3)2−
2 + H2O - 3 HON(SO
3)2−
2 + MnO−
4 + H+ → 3 ON(SO
3)2−
2 + MnO2 + 2 H2O - 2 ON(SO
3)2−
2 + 4 K+ → K4[ON(SO3)2]2
歷史
參考資料
擴展閱讀
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads