Loading AI tools
化合物 来自维基百科,自由的百科全书
鋯鈦酸鉛,是一種無機化合物,化學式Pb[Zr
xTi
1-x]O
3(0≤x≤1),通常縮寫為PZT(Lead Zirconate Titanate),是一種白色至灰白色固體。鋯鈦酸鉛是一種有明顯壓電效應的陶瓷鈣鈦礦材料,這意味着當施加電場時該化合物會改變形狀,被用於超聲波換能器、諧振器等。
| 鋯鈦酸鉛 | |
|---|---|
| |
| IUPAC名 鋯鈦酸鉛 | |
| 別名 | 鈦鋯酸鉛 |
| 識別 | |
| CAS號 | 12626-81-2 |
| PubChem | 159452 |
| SMILES |
|
| 性質 | |
| 化學式 | Pb[Zr xTi 1-x]O 3 (0≤x≤1) |
| 摩爾質量 | 303.065 ~ 346.4222 g/mol g·mol⁻¹ |
| 危險性 | |
GHS危險性符號  
| |
| GHS提示詞 | 危險 |
| H-術語 | H302, H332, H360, H373, H410 |
| P-術語 | P201, P202, P260, P261, P264, P270, P271, P273, P281, P301+312, P304+312, P304+340, P308+313, P312 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
鋯鈦酸鉛於1952年東京工業大學發現。與先前發現有明顯壓電效應的鈦酸鋇相比,鋯鈦酸鉛表現出更高靈敏度並具更好(較高)的工作溫度。陶瓷鋯鈦酸鉛因其物理強度、化學惰性、相對較低的製造成本而廣泛應用。陶瓷鋯鈦酸鉛是最常用的壓電陶瓷。[1]最近幾年,許多國家立法限制商業產品使用鉛,因此不少人尋找鋯鈦酸鉛的替代品。[2]
Being piezoelectric, lead zirconate titanate develops a voltage (or potential difference) across two of its faces when compressed (useful for sensor applications), and physically changes shape when an external electric field is applied (useful for actuator applications). The relative permittivity of lead zirconate titanate can range from 300 to 20000, depending upon orientation and doping.[來源請求]
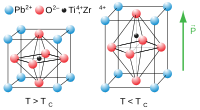
Being pyroelectric, this material develops a voltage difference across two of its faces under changing temperature conditions; consequently, lead zirconate titanate can be used as a heat sensor. Lead zirconate titanate is also ferroelectric, which means that it has a spontaneous electric polarization (electric dipole) that can be reversed in the presence of an electric field.
The material features an extremely large relative permittivity at the morphotropic phase boundary (MPB) near x = 0.52.[3]
Some formulations are ohmic until at least 250 kV/cm (25 MV/米), after which current grows exponentially with field strength before reaching avalanche breakdown; but lead zirconate titanate exhibits time-dependent dielectric breakdown — breakdown may occur under constant-voltage stress after minutes or hours, depending on voltage and temperature, so its dielectric strength depends on the time scale over which it is measured.[4] Other formulations have dielectric strengths measured in the 8–16 MV/米 range.[5]

Lead zirconate titanate-based materials are components of ceramic capacitors and STM/AFM actuators (tubes).
Lead zirconate titanate is used to make ultrasound transducers and other sensors and actuators, as well as high-value ceramic capacitors and FRAM chips. Lead zirconate titanate is also used in the manufacture of ceramic resonators for reference timing in electronic circuitry. In 1975 Sandia National Laboratories created anti-flash goggles featuring PLZT to protect aircrew from burns and blindness in case of a nuclear explosion.[6] The PLZT lenses could turn opaque in less than 150 microseconds.
Commercially, it is usually not used in its pure form, rather it is doped with either acceptors, which create oxygen (anion) vacancies, or donors, which create metal (cation) vacancies and facilitate domain wall motion in the material. In general, acceptor doping creates hard lead zirconate titanate, while donor doping creates soft lead zirconate titanate. Hard and soft lead zirconate titanate generally differ in their piezoelectric constants. Piezoelectric constants are proportional to the polarization or to the electrical field generated per unit of mechanical stress, or alternatively is the mechanical strain produced by per unit of electric field applied. In general, soft lead zirconate titanate has a higher piezoelectric constant, but larger losses in the material due to internal friction. In hard lead zirconate titanate, domain wall motion is pinned by the impurities, thereby lowering the losses in the material, but at the expense of a reduced piezoelectric constant.
One of the commonly studied chemical composition is PbZr
0.52Ti
0.48O
3. The increased piezoelectric response and poling efficiency near to x = 0.52 is due to the increased number of allowable domain states at the MPB. At this boundary, the 6 possible domain states from the tetragonal phase ⟨100⟩ and the 8 possible domain states from the rhombohedral phase ⟨111⟩ are equally favorable energetically, thereby allowing a maximum 14 possible domain states.
Like structurally similar lead scandium tantalate and barium strontium titanate, lead zirconate titanate can be used for manufacture of uncooled staring array infrared imaging sensors for thermographic cameras. Both thin film (usually obtained by chemical vapor deposition) and bulk structures are used. The formula of the material used usually approaches Pb
1.1(Zr
0.3Ti
0.7)O
3 (called lead zirconate titanate 30/70). Its properties may be modified by doping it with lanthanum, resulting in lanthanum-doped lead zirconium titanate (lead zirconate titanate, also called lead lanthanum zirconium titanate), with formula Pb
0.83La
0.17(Zr
0.3Ti
0.7)
0.9575O
3 (Lead zirconate titanate 17/30/70).[7]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.