赤堀氨基酸反应
来自维基百科,自由的百科全书
赤堀氨基酸反应(Akabori amino acid reaction)有两种:

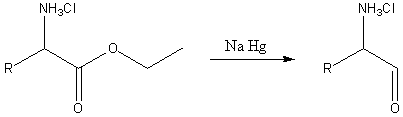
此条目翻译品质不佳。 (2021年4月16日) |
There are several Akabori amino acid reactions, which are named after Shirō Akabori (jap. 赤堀 四郎) (1900–1992), a Japanese chemist.
In the first reaction, an α-amino acid is oxidised and undergoes decarboxylation to give an aldehyde at the former α position by heating with oxygen in the presence of a reducing sugar.[1][2] This reaction is useful for preparing dichlorophthalimido derivatives[具体情况如何?] of peptides for mass spectral analysis.[3]
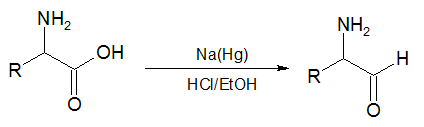
In the second reaction, an α-amino acid, or an ester of it, is reduced by sodium amalgam and ethanolic HCl to give an α-amino aldehyde.[4][5] This process is conceptually similar to the Bouveault–Blanc reduction[6][7][8] except that it stops at the aldehyde stage rather than reducing the ester all the way to two alcohols.
参见
参考资料
参考资料
Wikiwand - on
Seamless Wikipedia browsing. On steroids.