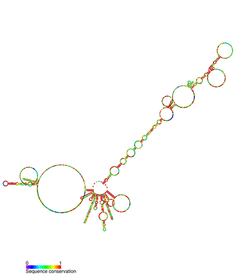
端粒酶RNA组分(英语:telomerase RNA component,缩写为TERC)或称端粒酶RNA,是一种真核生物的非编码RNA(ncRNA)。它是端粒酶的组成成分之一——端粒酶用它来延长端粒[2][3]。端粒酶RNA在DNA复制过程中,为端粒的逆转录过程提供模板。酵母、纤毛虫、脊椎动物的端粒酶RNA的序列和结构都有很大不同。不过,它们的5'端靠近模板序列处,都有一个假结结构。脊椎动物端粒酶RNA的3'端有一个与snoRNA(核仁RNA)类似的结构域[4][5][6]。
Quick Facts 端粒酶RNA组分 Telomerase RNA component (TERC), 标识 ...
Close
Quick Facts 脊椎动物端粒酶RNA, 识别符 ...
Close
Quick Facts 纤毛虫端粒酶RNA, 识别符 ...
Close
Quick Facts 酿酒酵母端粒酶RNA, 识别符 ...
Close
端粒酶系一类核糖核蛋白聚合酶。它通过给端粒加上重复序列来维持其长度。该重复序列在不同真核生物间有很大不同(具体可参见端粒条目中的比较表)。端粒酶除RNA外,还含有蛋白质组分(TERT,端粒酶逆转录酶)。端粒酶的蛋白质组分具有逆转录酶的活性。端粒酶的RNA组分,即端粒酶RNA,充当逆转录的模板。在脊椎动物端粒酶序列中,作为模板的CCCUAA序列在50位左右出现。端粒酶的表达在细胞衰老中扮演重要角色,在成熟体细胞中,端粒酶的表达通常处于受抑制状态,因而端粒会不断缩短。体细胞的端粒酶表达失调可能会诱发癌变。对小鼠的研究表明,端粒酶亦参与染色体修复,因为在双链都损坏的情况下,可能需要从头合成端粒重复序列[7]。在疱疹病毒亦发现了与端粒酶RNA同源的RNA[8]。
编码端粒酶RNA的基因的突变会造成造成常染色体显性遗传的先天性角化不良,也有可能与某些类型的再生障碍性贫血有关[7]。
Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. September 1995, 269 (5228): 1236–41. PMID 7544491. doi:10.1126/science.7544491. Lingner, J; Hendrick LL; Cech TR. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994, 8 (16): 1984–1998. PMID 7958872. doi:10.1101/gad.8.16.1984. Fragnet, L; Kut E; Rasschaert D. Comparative functional study of the viral telomerase RNA based on natural mutations. J Biol Chem. 2005, 280 (25): 23502–23515. PMID 15811851. doi:10.1074/jbc.M501163200.
- de Lange T, Jacks T. For better or worse? Telomerase inhibition and cancer. Cell. 1999, 98 (3): 273–5. PMID 10458601. doi:10.1016/S0092-8674(00)81955-8.
- Marrone A, Dokal I. Dyskeratosis congenita: molecular insights into telomerase function, ageing and cancer. Expert Reviews in Molecular Medicine. 2007, 6 (26): 1–23. PMID 15613268. doi:10.1017/S1462399404008671.
- Yamaguchi H. Mutations of telomerase complex genes linked to bone marrow failures. Journal of Nippon Medical School = Nihon Ika Daigaku zasshi. 2007, 74 (3): 202–9. PMID 17625368. doi:10.1272/jnms.74.202.
- Zaug AJ, Linger J, Cech TR. Method for determining RNA 3' ends and application to human telomerase RNA. Nucleic Acids Res. 1996, 24 (3): 532–3. PMC 145649
 . PMID 8602368. doi:10.1093/nar/24.3.532.
. PMID 8602368. doi:10.1093/nar/24.3.532.
- Soder AI, Hoare SF, Muire S, et al. Mapping of the gene for the mouse telomerase RNA component, Terc, to chromosome 3 by fluorescence in situ hybridization and mouse chromosome painting. Genomics. 1997, 41 (2): 293–4. PMID 9143511. doi:10.1006/geno.1997.4621.
- Zhao JQ, Hoare SF, McFarlane R, et al. Cloning and characterization of human and mouse telomerase RNA gene promoter sequences. Oncogene. 1998, 16 (10): 1345–50. PMID 9546436. doi:10.1038/sj.onc.1201892.
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999, 402 (6761): 551–5. PMID 10591218. doi:10.1038/990141.
- Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000, 100 (5): 503–14. PMID 10721988. doi:10.1016/S0092-8674(00)80687-X.
- Wong KK, Chang S, Weiler SR, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 2000, 26 (1): 85–8. PMID 10973255. doi:10.1038/79232.
- Mitchell JR, Collins K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell. 2000, 6 (2): 361–71. PMID 10983983. doi:10.1016/S1097-2765(00)00036-8.
- Imoto I, Pimkhaokham A, Fukuda Y, et al. SNO is a probable target for gene amplification at 3q26 in squamous-cell carcinomas of the esophagus. Biochem. Biophys. Res. Commun. 2001, 286 (3): 559–65. PMID 11511096. doi:10.1006/bbrc.2001.5428.
- Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001, 413 (6854): 432–5. PMID 11574891. doi:10.1038/35096585.
- Pruzan R, Pongracz K, Gietzen K, et al. Allosteric inhibitors of telomerase: oligonucleotide N3′→P5′ phosphoramidates. Nucleic Acids Res. 2002, 30 (2): 559–68. PMC 99832
 . PMID 11788719. doi:10.1093/nar/30.2.559.
. PMID 11788719. doi:10.1093/nar/30.2.559.
- Zhang RG, Zhang RP, Wang XW, Xie H. Effects of cisplatin on telomerase activity and telomere length in BEL-7404 human hepatoma cells. Cell Res. 2004, 12 (1): 55–62. PMID 11942411. doi:10.1038/sj.cr.7290110.
- Yang Y, Chen Y, Zhang C, et al. Nucleolar localization of hTERT protein is associated with telomerase function. Exp. Cell Res. 2002, 277 (2): 201–9. PMID 12083802. doi:10.1006/excr.2002.5541.
- Chang JT, Chen YL, Yang HT, et al. Differential regulation of telomerase activity by six telomerase subunits. Eur. J. Biochem. 2002, 269 (14): 3442–50. PMID 12135483. doi:10.1046/j.1432-1033.2002.03025.x.
- Gavory G, Farrow M, Balasubramanian S. Minimum length requirement of the alignment domain of human telomerase RNA to sustain catalytic activity in vitro. Nucleic Acids Res. 2002, 30 (20): 4470–80. PMC 137139
 . PMID 12384594. doi:10.1093/nar/gkf575.
. PMID 12384594. doi:10.1093/nar/gkf575.
- Sood AK, Coffin J, Jabbari S, et al. p53 null mutations are associated with a telomerase negative phenotype in ovarian carcinoma. Cancer Biol. Ther. 2003, 1 (5): 511–7. PMID 12496479. doi:10.4161/cbt.1.5.167.
- Antal, M; Boros E; Solymosy F; Kiss T. Analysis of the structure of human telomerase RNA in vivo. Nucleic Acids Res. 2002, 30 (4): 912–920. PMC 100349
 . PMID 11842102. doi:10.1093/nar/30.4.912.
. PMID 11842102. doi:10.1093/nar/30.4.912.