From Wikipedia, the free encyclopedia
Radioactive decay, cried nuclear decay or radioactivity an aa, is the process bi whilk a nucleus o an unstable atom loses virr bi ootpittin pairticles o ionisin radiation. A material that spontaneously ootpits this kin o radiation—whilk includes the emission o energetic alpha pairticles, beta pairticles, an gamma rays—is conseidered radioactive. Certaint heichly excited short-lived nuclear states can decay throu neutron emission, or mair rarely, proton emission.
The "Scots" that wis uised in this airticle wis written bi a body that haesna a guid grip on the leid. Please mak this airticle mair better gin ye can. (September 2020) |
Radioactive decay is a stochastic (i.e. random) process at the level o single atoms, in that, accordin tae quantum theory, it is impossible tae predict when a parteecular atom will decay,[1][2][3][4] regairdless o how lang the atom haes existit. Houiver, for a collection o atoms, the collection's expected decay rate is characterized in terms o thair measured decay constants or hauf-lifes. This is the basis o radiometric dating. The hauf-lifes o radioactive atoms hae na kent upper leemit, spannin a time range o ower 55 orders o magnitude, frae nearly instantaneous tae far langer than the age o the universe.
A radioactive nucleus wi zero spin can hae no defined orientation, an hence emits the tot momentum o its decay products isotropically (aw directions an wioot bias). If thare are multiple particles produced in a single decay, as in beta decay, thair relative angular distribution, or spin directions mey nae be isotropic. Decay products frae a nucleus wi spin mey be distributed non-isotropically wi respect tae that spin direction, aither acause o an external influence sic as an electromagnetic field, or acause the nucleus wis produced in a dynamic process that constrained the direction o its spin. Sic a parent process coud be a previous decay, or a nuclear reaction.[5][6][7][note 1]
The decaying nucleus is cried the parent radionuclide (or parent radioisotope[note 2]), an the process produces at least ane daughter nuclide. Except for gamma decay or internal conversion frae a nuclear excited state, the decay is a nuclear transmutation resultin in a dochter conteenin a different nummer o protons or neutrons (or baith). When the nummer o protons changes, an atom o a different chemical element is creatit.
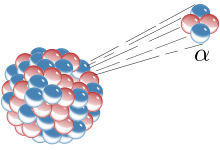
The first decay processes tae be discovered war alpha decay, beta decay, an gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the maist common process o emittin nucleons, but heichly excited nuclei can eject single nucleons, or in the case o cluster decay, specific licht nuclei o ither elements. Beta decay occurs when the nucleus emits an electron or positron an a neutrino, in a process that changes a proton tae a neutron or the converse. Highly excited neutron-rich nuclei, formed as the product o ither teeps o decay, occasionally lose energy bi wey o neutron emission, resultin in a chynge frae ane isotope tae anither o the same element. The nucleus mey captur an orbitin electron, causin a proton tae convert intae a neutron in a process cried electron captur. Aw o thir processes result in a well-defined nuclear transmutation.
Bi contrast, thare are radioactive decay processes that dae nae result in a nuclear transmutation. The energy o an excitit nucleus mey be emitted as a gamma ray in a process cried gamma decay, or that energy mey be lost when the nucleus interacts wi an orbital electron causin its ejection frae the atom, in a process cried internal conversion.
Anither teep o radioactive decay results in products that vary, appearin as twa or mair "fragments" o the oreeginal nucleus wi a range o possible masses. This decay, cried spontaneous fission, happens when a lairge unstable nucleus spontaneously splits intae twa (or occasionally three) smawer dochter nuclei, an generally leads tae the emission o gamma rays, neutrons, or ither particles frae those products.
For a summary table shawin the nummer o stable an radioactive nuclides in each category, see radionuclide. Thare are 29 naturally occurrin chemical elements on Yird that are radioactive. Thay are those that contain 34 radionuclides that date afore the time o formation o the solar seestem, an are kent as primordial nuclides. Well-kent examples are uranium an thorium, but forby included are naiturally occurrin lang-lived radioisotopes, sic as potassium-40. Anither 50 or sae shorter-lived radionuclides, sic as radium an radon, foond on Yird, are the products o decay cheens that began wi the primordial nuclides, or are the product o ongaein cosmogenic processes, sic as the production o carbon-14 frae nitrogen-14 in the atmosphere bi cosmic rays. Radionuclides mey forby be produced artificially in particle accelerators or nuclear reactors, resultin in 650 o thir wi hauf-lifes o ower an oor, an several thoosand mair wi even shorter hauf-lifes. [See here for a leet o thir sorted bi hauf life.]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.