Top Qs
Timeline
Chat
Perspective
Phosphonium
Family of polyatomic cations containing phosphorus From Wikipedia, the free encyclopedia
Remove ads
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula PR+
4 (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.[1]
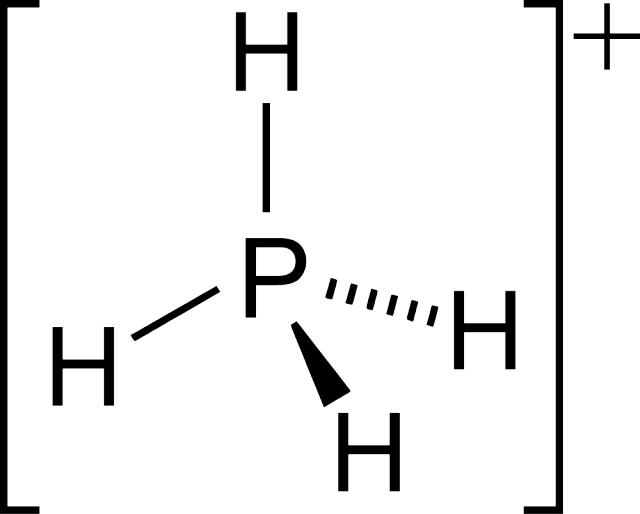

4, the parent phosphonium cation.
Types of phosphonium cations
Summarize
Perspective
Protonated phosphines
The parent phosphonium is PH+
4 as found in the iodide salt, phosphonium iodide. Salts of the parent PH+
4 are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride:
- PH3 + HCl + 4 CH2O → P(CH
2OH)+
4Cl−
Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines:
- PR3 + H+ → HPR+
3
The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl.[2]
Tetraorganophosphonium cations
The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphonium P(CH
3)+
4.


Quaternary phosphonium cations (PR+
4) are produced by alkylation of organophosphines.[3] For example, the reaction of triphenylphosphine with methyl bromide gives methyltriphenylphosphonium bromide:
- PPh3 + CH3Br → [CH3PPh3]+Br−
The methyl group in such phosphonium salts is mildly acidic, with a pKa estimated to be near 15:[5]
- [CH3PPh3]+ + base → CH2=PPh3 + [Hbase]+
This deprotonation reaction gives Wittig reagents.[6]
Phosphorus pentachloride and related compounds
Solid phosphorus pentachloride is an ionic compound, formulated [PCl4]+[PCl6]− (tetrachlorophosphonium hexachlorophosphate(V)), that is, a salt containing the tetrachlorophosphonium cation.[7][8] Dilute solutions dissociate according to the following equilibrium:
- PCl5 ⇌ PCl+
4 + Cl−
Triphenylphosphine dichloride (Ph3PCl2) exists both as the pentacoordinate phosphorane and as the chlorotriphenylphosphonium chloride, depending on the medium.[9] The situation is similar to that of PCl5. It is an ionic compound (PPh3Cl)+Cl− in polar solutions and a molecular species with trigonal bipyramidal molecular geometry in apolar solution.[10]
Alkoxyphosphonium salts: Arbuzov reaction
The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. Commonly, the phosphorus substrate is a phosphite ester (P(OR)3) and the alkylating agent is an alkyl iodide.[11]

Remove ads
Uses
Summarize
Perspective
Textile finishes

Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and flame-retardant finishes on cotton textiles and other cellulosic fabrics.[12][13] A flame-retardant finish can be prepared from THPC by the Proban Process,[14] in which THPC is treated with urea. The urea condenses with the hydroxymethyl groups on THPC. The phosphonium structure is converted to phosphine oxide as the result of this reaction.[15]
Phase-transfer catalysts and precipitating agents
Organic phosphonium cations are lipophilic and can be useful in phase transfer catalysis, much like quaternary ammonium salts. Salts or inorganic anions and tetraphenylphosphonium (PPh+
4) are soluble in polar organic solvents. One example is the perrhenate (PPh4[ReO4]).[16]
Reagents for organic synthesis
Wittig reagents are used in organic synthesis. They are derived from phosphonium salts. A strong base such as butyllithium or sodium amide is required for the deprotonation:
- [Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H10
One of the simplest ylides is methylenetriphenylphosphorane (Ph3P=CH2).[6]
The compounds Ph3PX2 (X = Cl, Br) are used in the Kirsanov reaction.[17] The Kinnear–Perren reaction is used to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and esters (RP(O)(OR′)2). A key intermediate are alkyltrichlorophosphonium salts, obtained by the alkylation of phosphorus trichloride:[18]
- RCl + PCl3 + AlCl3 → [RPCl3]+AlCl−
4
Ammonia production for "green hydrogen"
The main industrial procedure for the production of ammonia today is the thermal Haber-Bosch process, which generally uses fossil gas as a source of hydrogen, which is then combined with nitrogen to produce ammonia. In 2021, Professor Doug MacFarlane and collaborators Alexandr Simonov and Bryan Suryanto of Monash University devised a method of producing green ammonia that has the potential to make Haber-Bosch plants obsolete.[19] Their process is similar to the electrolysis approach for producing hydrogen. While working with local company Verdant, which wanted to make bleach from saltwater by electrolysis, Suryanto discovered that a tetraalkyl phosphonium salt allowed the efficient production of ammonia at room temperature.[20]
Remove ads
See also
- Ammonium (NH+
4) - Arsonium (AsH+
4) - Hydronium (H3O+)
- Onium compounds
- Organophosphorus chemistry
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
