Loading AI tools
Combination of two antibiotic drugs From Wikipedia, the free encyclopedia
Trimethoprim/sulfamethoxazole, sold under the brand name Bactrim among others, is a fixed-dose combination antibiotic medication used to treat a variety of bacterial infections.[2] It consists of one part trimethoprim to five parts sulfamethoxazole.[7] It is used to treat urinary tract infections, methicillin-resistant Staphylococcus aureus (MRSA) skin infections, travelers' diarrhea, respiratory tract infections, and cholera, among others.[2][7] It is used both to treat and prevent pneumocystis pneumonia and toxoplasmosis in people with HIV/AIDS and other causes of immunosuppression.[2] It can be given orally (swallowed by mouth) or intravenous infusion (slowly injected into a vein with an IV).[2]
 Trimethoprim (top) and sulfamethoxazole (bottom) | |
| Combination of | |
|---|---|
| Sulfamethoxazole | Sulfonamide antibiotic |
| Trimethoprim | Dihydrofolate reductase inhibitor |
| Clinical data | |
| Trade names | Bactrim, Cotrim, Septra, others |
| Other names | TMP/SMX, cotrimoxazole, Co-trimoxazole (BAN UK) |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | oral, intravenous[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| (verify) | |
Trimethoprim/sulfamethoxazole is on the World Health Organization's List of Essential Medicines.[8] It is available as a generic medication.[7][9] In 2022, it was the 143rd most commonly prescribed medication in the United States, with more than 3 million prescriptions.[10][11]
Trimethoprim/sulfamethoxazole generally kills bacteria,[2] by blocking the microorganisms' ability to make and to use folate.[2]
Trimethoprim/sulfamethoxazole (TMP/SMX) is the medicine most commonly used to prevent Pneumocystis jirovecii pneumonia (PCP)[12] People who get Pneumocystis pneumonia have a medical condition that weakens their immune system, like HIV/AIDS, or take medicines (such as corticosteroid,mono clonal antibody,immunosuppressants) that reduce the body's ability to fight bacterial and viral infections. People with HIV/AIDS are less likely to get Pneumocystis pneumonia as a result of antiretroviral therapy (ART). However, Pneumocystis pneumonia is still a substantial public health problem. Most of what is scientifically known about Pneumocystis pneumonia and its treatment comes from studying people with HIV/AIDS.[12]
Organisms against which trimethoprim/sulfamethoxazole can be effective include:[13][14]
The only notable nonsusceptible organisms are Pseudomonas aeruginosa, the mycoplasmae[14] and Francisella tularensis (the causative organism of tularaemia).[15][16]
Its use during pregnancy is contraindicated, although it has been placed in Australian pregnancy category C.[13] Its use during the first trimester (during organogenesis) and 12 weeks prior to pregnancy has been associated with an increased risk of congenital malformations, especially malformations associated with maternal folic acid deficiency (which is most likely related to the mechanism of action of co-trimoxazole) such as neural tube defects such as spina bifida, cardiovascular malformations (e.g. Ebstein's anomaly), urinary tract defects, oral clefts, and club foot in epidemiological studies.[13] Its use later on during pregnancy also increases the risk of preterm labour (odds ratio: 1.51) and low birth weight (odds ratio: 1.67).[17][18] Animal studies have yielded similarly discouraging results.[3]
It appears to be safe for use during breastfeeding as long as the baby is healthy.[19]
Its use in those less than 2 months of age is not recommended due to the risk of adverse side effects.[20]
Common side effects include nausea, vomiting, rash, and diarrhea.[2] Severe allergic reactions and Clostridioides difficile infection may occasionally occur.[2] Its use in pregnancy is not recommended.[2][19] It appears to be safe for use during breastfeeding as long as the baby is healthy.[19]
Contraindications include the following:[13][5]
Its use is advised against in people being concomitantly treated with:[13][3][5][6][21][22]
Likely signs of toxicity include:[3]
The recommended treatment for overdose includes:[3]
Alkalinisation of the urine may reduce the toxicity of sulfamethoxazole, but it may increase the toxic effects of trimethoprim.[3]
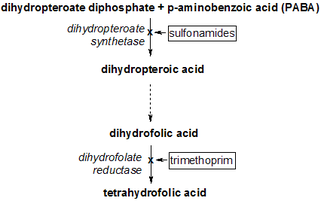
The synergy between trimethoprim and sulfamethoxazole was first described in the late 1960s.[25][26][27] Trimethoprim and sulfamethoxazole have a greater effect when given together than when given separately, because they inhibit successive steps in the folate synthesis pathway. They are given in a one-to-five ratio in their tablet formulations so that when they enter the body their concentration in the blood and tissues is roughly one-to-twenty — the exact ratio required for a peak synergistic effect between the two.[14]
Sulfamethoxazole, a sulfonamide, induces its therapeutic effects by interfering with the de novo (that is, from within the cell) synthesis of folate inside microbial organisms such as protozoa, fungi and bacteria. It does this by competing with p-aminobenzoic acid (PABA) in the biosynthesis of dihydrofolate.[14]
Trimethoprim serves as a competitive inhibitor of dihydrofolate reductase (DHFR), hence inhibiting the de novo synthesis of tetrahydrofolate, the biologically active form of folate.[14]
Tetrahydrofolate is crucial in the synthesis of purines, thymidine, and methionine which are needed for the production of DNA and proteins[28] during bacterial replication.
The effects of trimethoprim causes a backlog of dihydrofolate (DHF) and this backlog can work against the inhibitory effect the drug has on tetrahydrofolate biosynthesis. This is where the sulfamethoxazole comes in; its role is in depleting the excess DHF by preventing it from being synthesised in the first place.[14]
Co-trimoxazole was claimed to be more effective than either of its components individually in treating bacterial infections, although this was later disputed.[29][30]
| Component | Tmax (h) | Vd (L) | Protein binding | t1/2 (h) | Excretion |
|---|---|---|---|---|---|
| Sulfamethoxazole | 1-4 | 20 | 66% | 8-10 | Renal |
| Trimethoprim | 1-4 | 130 | 42-45% | 10 | Renal |
| Indication | FDA-labelled indication? |
TGA-labelled indication? |
MHRA-labelled indication? |
Literature support |
|---|---|---|---|---|
| Acute infective exacerbation of COPD | Yes | No | No | Clinical trials are lacking. |
| Prophylaxis in HIV-infected individuals | No | No | No | Effective in one Ugandan study on morbidity, mortality, CD4-cell count, and viral load in HIV infection.[31] |
| Otitis media | Paediatric population only | No | Yes | Clinical trials have confirmed its efficacy in chronic active otitis media[32] and acute otitis media.[33] |
| Travellers' diarrhea, treatment & prophylaxis | Yes | No | No | Clinical trials have confirmed its efficacy as a treatment for travellers' diarrhea.[34][35][36] |
| Urinary tract infection | Yes | No | Yes | Clinical trials have confirmed its efficacy in this indication.[14] |
| Bacterial infections | ||||
| Acne vulgaris | No | No | No | At least one clinical trial supports its use in this indication.[37] |
| Listeria | No | Yes | No | Well-designed clinical trials are lacking. |
| Melioidosis | No | Yes | No | Clinical trials have confirmed its efficacy, with or without adjunctive doxycycline; although, co-trimoxazole alone seems preferable.[38][39][40] |
| Pertussis (whooping cough) | No | No | No | One cochrane review supports its efficacy in preventing the spread of pertussis.[41] |
| Shigellosis | Yes | Yes | No | Generally accepted treatment for shigellosis.[42] A recent Cochrane review found that while it is an effective treatment for shigellosis it also produces more significant adverse effects than other antibiotic drugs.[43] |
| Staphylococcus aureus infections | No | No | No | In vitro and in vivo activity against both non-resistant and methicillin-resistant Staphylococcus aureus (MRSA) infections.[44][45][46][47][48][49][50] |
| Tuberculosis | No | No | No | In vitro and in vivo activity against both nonresistant and MDR strains of TB.[51][52][53] |
| Whipple's disease | No | No | No | Co-trimoxazole is the recommended standard treatment for whipple's disease in some treatment protocols.[54][55][56] |
| Fungal and protozoal infections | ||||
| Isosporiasis | No | No | No | Clinical trials have confirmed its use in this indication.[57] |
| Malaria | No | No | No | Clinical trials have confirmed its efficacy in both the treatment and prevention of malaria.[58] |
| Pneumocystis jirovecii pneumonia | Yes | Yes | Yes | Its use as a prophylactic treatment is supported by one clinical trial involving children with acute lymphoblastic leukaemia.[59] Other than this and one other clinical trial into its efficacy as a treatment for pneumocystis pneumonia,[60] data on its use in both the treatment and prevention of pneumocystis pneumonia is significantly lacking. |
| Toxoplasmosis | Yes | Prevention only | Yes | Clinical trials have confirmed its prophylactic and therapeutic utility in cases of toxoplasmosis.[61][62][63][64][65][66] |
Trimethoprim/sulfamethoxazole may be abbreviated as SXT, SMZ-TMP, TMP-SMX, TMP-SMZ, or TMP-sulfa.[citation needed] The generic British Approved Name (BAN) Co-trimoxazole is used for trimethoprim/sulfamethoxazole manufactured and sold by many different companies.[67]
The following list of brand names is incomplete:
Trimethoprim/sulfamethoxazole is relatively inexpensive as of 2019.[9]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.