Top Qs
Timeline
Chat
Perspective
Spiro compound
Any chemical compound having one atom as the only common member of two rings From Wikipedia, the free encyclopedia
Remove ads
In organic chemistry, spiro compounds are compounds that have at least two molecular rings sharing one common atom. Simple spiro compounds are bicyclic (having just two rings).[2]: SP-0 [3]: 653, 839 The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics.[4][3]: 653ff : 839ff Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone.
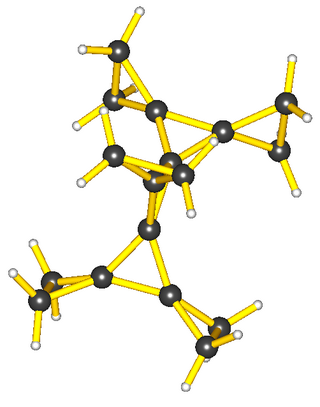
The common atom that connects the two (or sometimes three) rings is called the spiro atom.[2]: SP-0 In carbocyclic spiro compounds like spiro[5.5]undecane, the spiro-atom is a quaternary carbon, and as the -ane ending implies, these are the types of molecules to which the name spirane was first applied (though it is now used general of all spiro compounds).[5]: 1138ff The two rings sharing the spiro atom are most often different, although they can be identical [e.g., spiro[5.5]undecane and spiropentadiene, at right].[3]: 319f.846f
Remove ads
Selected spiro compounds
- Elatol, isolated from Laurencia dendroidea (red algae)[6]
- Spironolactone, a commercial diuretic medication
- Spiropentadiene, which is highly strained.[7]
- (A) 1-Bromo-3-chlorospiro[4.5]decan-7-ol, and (B) '1-bromo-3-chlorospiro[3.6]decan-7-ol.
Carbocyclic spiro compounds
Summarize
Perspective
Bicyclic ring structures in organic chemistry that have two fully carbocyclic (all carbon) rings connected through a carbon atom are the usual focus of the topic of spirocycles. Simple parent spirocycles include spiropentane, spirohexane, etc. up to spiroundecane. Several exist as isomers. Lower members of the class are strained. The symmetric isomer of spiroundecane is not.
Some spirocyclic compounds occur as natural products.[6]
Preparation


The spirocyclic core is usually prepared by dialkylation of an activated carbon center. The dialkylating group is often a 1,3-, 1,4-, etc. dihalide.[9] In some cases the dialkylating group is a dilithio reagent, such as 1,5-dilithiopentane.[10] For generating spirocycles containing a cyclopropane ring, cyclopropanation with cyclic carbenoids has been demonstrated.[11]
Spiro compounds are often prepared by diverse rearrangement reactions. For example, the pinacol-pinacolone rearrangement is illustrated below.[3]: 985 is employed in the preparation of aspiro[4.5]decane.[12]].

Remove ads
Heterocyclic spiro compounds

Spiro compounds are considered heterocyclic if the spiro atom or any atom in either ring are not carbon atoms. Cases with a spiro heteroatom such as boron, silicon, and nitrogen (but also other Group IVA [14] are often trivial to prepare. Many borate esters derived from glycols illustrate this case.[14] Likewise, a tetravalent neutral silicon and quaternary nitrogen atom (ammonium cation) can be the spiro center. Many such compounds have been described.[5]: 1139f
Particularly common spiro compounds are ketal (acetal) formed by condensation of cyclic ketones and diols and dithiols.[15][16][17] A simple case is the acetal 1,4-dioxaspiro[4.5]decane from cyclohexanone and glycol. Cases of such ketals and dithioketals are common.
Chirality
Summarize
Perspective

Spiranes can be chiral,[18] in various ways.[5]: 1138ff First, while nevertheless appearing to be twisted, they yet may have a chiral center making them analogous to any simple chiral compound, and second, while again appearing twisted, the specific location of substituents, as with alkylidenecycloalkanes, may make a spiro compound display central chirality (rather than axial chirality resulting from the twist); third, the substituents of the rings of the spiro compound may be such that the only reason they are chiral arises solely from the twist of their rings, e.g., in the simplest bicyclic case, where two structurally identical rings are attached via their spiro atom, resulting in a twisted presentation of the two rings.[5]: 1138ff, 1119ff [3]: 319f.846f Hence, in the third case, the lack of planarity described above gives rise to what is termed axial chirality in otherwise identical isomeric pair of spiro compounds, because they differ only in the right- versus left-handed "twist" of structurally identical rings (as seen in allenes, sterically hindered biaryls, and alkylidenecycloalkanes as well).[5]: 1119f Assignment of absolute configuration of spiro compounds has been challenging, but a number of each type have been unequivocally assigned.[5]: 1139ff
Some spiro compounds exhibit axial chirality. Spiroatoms can be the origin of chirality even when they lack the required four different substituents normally observed in chirality. When two rings are identical the priority is determined by a slight modification of the CIP system assigning a higher priority to one ring extension and a lower priority to an extension in the other ring. When rings are dissimilar the regular rules apply.[clarification needed]
Remove ads
Nomenclature and etymology
Nomenclature for spiro compounds was first discussed by Adolf von Baeyer in 1900.[19] IUPAC provides advice on naming of spiro compounds.[20]
The prefix spiro denotes two rings with a spiro junction. The main method of systematic nomenclature is to follow with square brackets containing the number of atoms in the smaller ring then the number of atoms in the larger ring, separated by a period, in each case excluding the spiroatom (the atom by which the two rings are bonded) itself. Position-numbering starts with an atom of the smaller ring adjacent to the spiroatom around the atoms of that ring, then the spiroatom itself, then around the atoms of the larger ring.[21] For example, compound A in Image #4 above (Selected Spiro Compounds) is called 1-bromo-3-chlorospiro[4.5]decan-7-ol, and compound B is called 1-bromo-3-chlorospiro[3.6]decan-7-ol.
Remove ads
Further reading
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford, UK: Oxford University Press. pp. 319f, 432, 604np, 653, 746int, 803ketals, 839, 846f. ISBN 978-0-19-927029-3. Retrieved 2 February 2016.
- Eliel, Ernest Ludwig; Wilen, Samuel H.; Mander, Lewis N. (1994). "Chirality in Molecules Devoid of Chiral Centers (Chapter 14)". Stereochemistry of Organic Compounds (1st ed.). New York, NY, USA: Wiley & Sons. pp. 1119–1190, esp. 1119ff, 1138ff, and passim. ISBN 978-0-471-01670-0. Retrieved 2 February 2016.
- Examples of spiro natural products and their synthesis: Smith, Laura K. & Baxendale, Ian R. (2015). "Total Syntheses of Natural Products Containing Spirocarbocycles". Org. Biomol. Chem. 13 (39): 9907–9933. doi:10.1039/C5OB01524C. PMID 26356301.
- Saragi, Tobat P. I.; Spehr, Till; Siebert, Achim; Fuhrmann-Lieker, Thomas; Salbeck, Josef (2007). "Spiro Compounds for Organic Optoelectronics". Chemical Reviews. 107 (4): 1011–1065. Bibcode:2007ChRv..107.1011S. doi:10.1021/cr0501341. PMID 17381160.
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads




