Samarium(III) antimonide
Chemical compound From Wikipedia, the free encyclopedia
Samarium antimonide is a binary inorganic compound of samarium and antimony with the formula SmSb. It forms crystals.
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.045.224 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PrSb | |
| Molar mass | 272.12 g/mol |
| Density | 7.3 g/cm3 |
| Melting point | 1922 °C |
| Related compounds | |
Other anions |
SmN, SmP, SmAs, SmBi, Sm2O3 |
Other cations |
PrSb, NdSb |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
Samarium antimonide can be prepared by heating samarium and antimony in a vacuum:
Physical properties
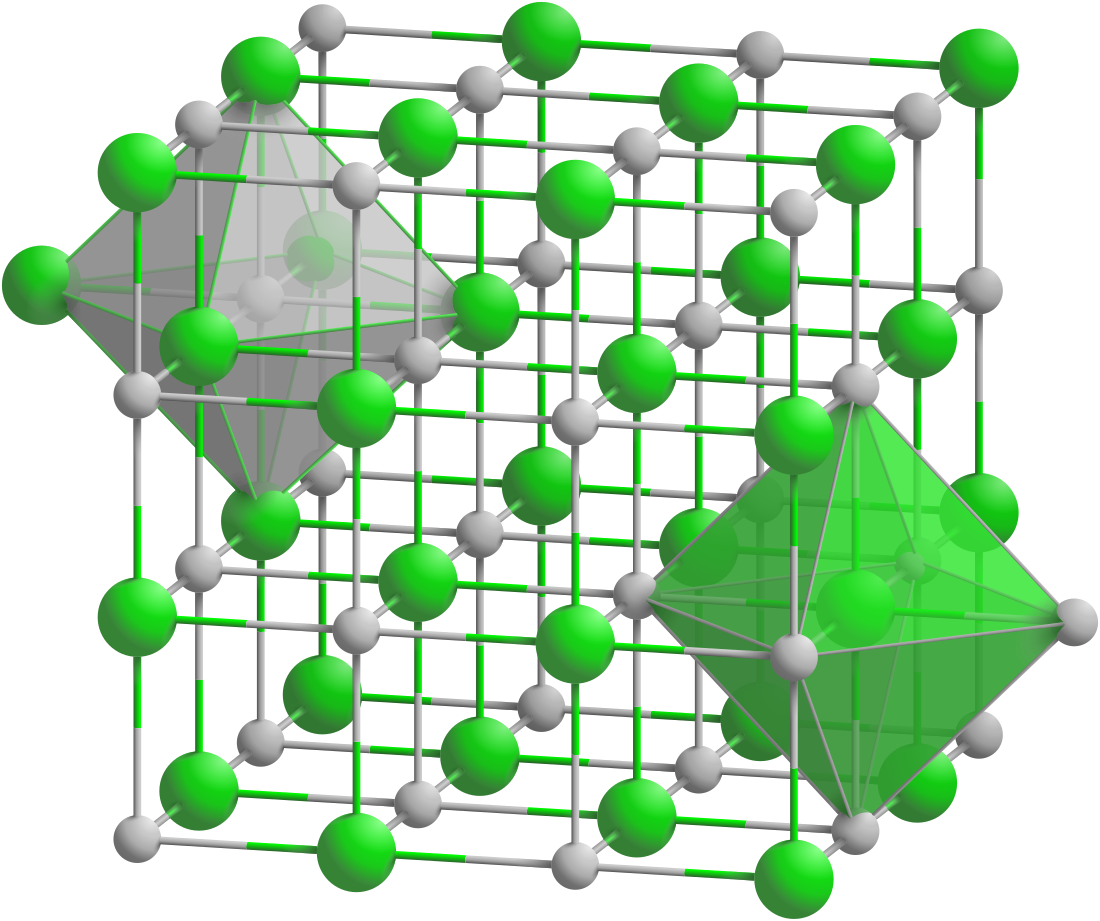
Samarium antimonide forms cubic crystals, space group Fm3m, cell parameters a = 0.6271 nm, Z = 4, and structure like sodium chloride.[1][2][3]
The compound melts congruently at a temperature of ≈2000 °C[1] or 1922 °C.[3]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.