Plutonium-241
Isotope of plutonium From Wikipedia, the free encyclopedia
Plutonium-241 (241
Pu
or Pu-241) is an isotope of plutonium formed when plutonium-240 captures a neutron. Like some other plutonium isotopes (especially 239Pu), 241Pu is fissile, with a neutron absorption cross section about one-third greater than that of 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission case, neutron capture produces plutonium-242. In general, isotopes with an odd number of neutrons are both more likely to absorb a neutron and more likely to undergo fission on neutron absorption than isotopes with an even number of neutrons.
This article needs additional citations for verification. (November 2021) |
| General | |
|---|---|
| Symbol | 241Pu |
| Names | plutonium-241, 241Pu, Pu-241 |
| Protons (Z) | 94 |
| Neutrons (N) | 147 |
| Nuclide data | |
| Natural abundance | 0 (Artificial) |
| Half-life (t1/2) | 14 years |
| Isotope mass | 241.057 Da |
| Decay products | 241Am 237U |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β− | 0.02078(17)[1] |
| α | 5.055(5)[1] |
| Isotopes of plutonium Complete table of nuclides | |
Decay properties
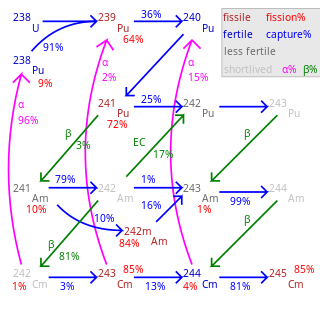
Plutonium-241 is a beta emitter with a half-life of 14.3 years, corresponding to a decay of about 5% of 241Pu nuclei over a one-year period. This decay has a Q-value of 20.78±0.17 keV and a mean of 5.227±0.043 keV, and does not emit gamma rays.[1] The longer spent nuclear fuel waits before reprocessing, the more 241Pu decays to americium-241, which is nonfissile (although fissionable by fast neutrons) and an alpha emitter with a half-life of 432 years; 241Am is a major contributor to the radioactivity of nuclear waste on a scale of hundreds or thousands of years.[citation needed] In its fully ionized state, the beta-decay half-life of 241Pu94+ decreases to 4.2 days, and only bound-state beta decay is possible.[2]
Plutonium-241 also has a rare alpha decay branch to uranium-237, occurring in about 0.002% of decays. With a Q-value of 5.055±0.005 MeV, it can emit Auger electrons and associated X-rays, unlike the beta-decay process.[1]
Role in nuclear fuel
Summarize
Perspective
Americium has lower valence and lower electronegativity than plutonium, neptunium or uranium, so in most nuclear reprocessing, americium tends to fractionate with the alkaline fission products – lanthanides, strontium, caesium, barium, yttrium – rather than with other actinides. Americium is therefore not recycled into nuclear fuel unless special efforts are made.
In a thermal reactor, 241Am captures a neutron to become americium-242, which quickly becomes curium-242 (or, 17.3% of the time, 242Pu) via beta decay. Both 242Cm and 242Pu are much less likely to absorb a neutron, and even less likely to fission; however, 242Cm is short-lived (half-life 160 days) and almost always undergoes alpha decay to 238Pu rather than capturing another neutron. In short, 241Am needs to absorb two neutrons before again becoming a fissile isotope.
| Actinides[3] by decay chain | Half-life range (a) |
Fission products of 235U by yield[4] | ||||||
|---|---|---|---|---|---|---|---|---|
| 4n | 4n + 1 | 4n + 2 | 4n + 3 | 4.5–7% | 0.04–1.25% | <0.001% | ||
| 228Ra№ | 4–6 a | 155Euþ | ||||||
| 248Bk[5] | > 9 a | |||||||
| 244Cmƒ | 241Puƒ | 250Cf | 227Ac№ | 10–29 a | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Puƒ | 243Cmƒ | 29–97 a | 137Cs | 151Smþ | 121mSn | ||
| 249Cfƒ | 242mAmƒ | 141–351 a |
No fission products have a half-life | |||||
| 241Amƒ | 251Cfƒ[6] | 430–900 a | ||||||
| 226Ra№ | 247Bk | 1.3–1.6 ka | ||||||
| 240Pu | 229Th | 246Cmƒ | 243Amƒ | 4.7–7.4 ka | ||||
| 245Cmƒ | 250Cm | 8.3–8.5 ka | ||||||
| 239Puƒ | 24.1 ka | |||||||
| 230Th№ | 231Pa№ | 32–76 ka | ||||||
| 236Npƒ | 233Uƒ | 234U№ | 150–250 ka | 99Tc₡ | 126Sn | |||
| 248Cm | 242Pu | 327–375 ka | 79Se₡ | |||||
| 1.33 Ma | 135Cs₡ | |||||||
| 237Npƒ | 1.61–6.5 Ma | 93Zr | 107Pd | |||||
| 236U | 247Cmƒ | 15–24 Ma | 129I₡ | |||||
| 244Pu | 80 Ma |
... nor beyond 15.7 Ma[7] | ||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7–14.1 Ga | |||||
| ||||||||
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.