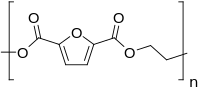
Polyethylene furan-2,5-dicarboxylate
Chemical compound From Wikipedia, the free encyclopedia
Polyethylene furan-2,5-dicarboxylate, also named poly(ethylene furan-2,5-dicarboxylate), polyethylene furanoate and poly(ethylene furanoate) and generally abbreviated as PEF, is a polymer that can be produced by polycondensation or ring-opening polymerization of 2,5-furandicarboxylic acid (FDCA) and ethylene glycol.[2][3] As an aromatic polyester from ethylene glycol it is a chemical analogue of polyethylene terephthalate (PET) and polyethylene naphthalate (PEN). PEF has been described in (patent) literature since 1951,[4] but has gained renewed attention since the US department of energy proclaimed its building block, FDCA, as a potential bio-based replacement for purified terephthalic acid (PTA) in 2004.[5]
 | |
| Names | |
|---|---|
| Other names
Polyethylene furanoate; Polyethylene furandicarboxylate; Poly(ethylene furanoate) | |
| Identifiers | |
| Properties | |
| (C8H6O5)n | |
| Molar mass | Variable |
| Density | 1.43 g/cm3[1][2] |
| Melting point | 195–265 °C (383–509 °F; 468–538 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benefits over PET
One life-cycle assessment showed that replacing PTA in the production of PET by bio-based FDCA for the production of PEF has a potential for significant reductions in greenhouse gas (GHG) emissions and non-renewable energy use (NREU).[6] Furthermore, PEF exhibits an intrinsically higher gas barrier for oxygen,[7] carbon dioxide[8] and water vapor[9] than PET and is therefore an interesting alternative for packaging applications such as bottles, films and food trays.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
