Loading AI tools
Asexual reproduction without fertilization From Wikipedia, the free encyclopedia
Parthenogenesis (/ˌpɑːrθɪnoʊˈdʒɛnɪsɪs, -θɪnə-/;[1][2] from the Greek παρθένος, parthénos, 'virgin' + γένεσις, génesis, 'creation'[3]) is a natural form of asexual reproduction in which the embryo develops directly from an egg without need for fertilization. In animals, parthenogenesis means development of an embryo from an unfertilized egg cell. In plants, parthenogenesis is a component process of apomixis. In algae, parthenogenesis can mean the development of an embryo from either an individual sperm or an individual egg.

Parthenogenesis occurs naturally in some plants, algae, invertebrate animal species (including nematodes, some tardigrades, water fleas, some scorpions, aphids, some mites, some bees, some Phasmatodea, and parasitic wasps), and a few vertebrates, such as some fish, amphibians, and reptiles. This type of reproduction has been induced artificially in animal species that naturally reproduce through sex, including fish, amphibians, and mice.
Normal egg cells form in the process of meiosis and are haploid, with half as many chromosomes as their mother's body cells. Haploid individuals, however, are usually non-viable, and parthenogenetic offspring usually have the diploid chromosome number. Depending on the mechanism involved in restoring the diploid number of chromosomes, parthenogenetic offspring may have anywhere between all and half of the mother's alleles. In some types of parthenogenesis the offspring having all of the mother's genetic material are called full clones and those having only half are called half clones. Full clones are usually formed without meiosis. If meiosis occurs, the offspring get only a fraction of the mother's alleles since crossing over of DNA takes place during meiosis, creating variation.
Parthenogenetic offspring in species that use either the XY or the X0 sex-determination system have two X chromosomes and are female. In species that use the ZW sex-determination system, they have either two Z chromosomes (male) or two W chromosomes (mostly non-viable but rarely a female), or they could have one Z and one W chromosome (female).

Parthenogenesis is a form of asexual reproduction in which the embryo develops directly from an egg without need for fertilization.[4][5] It occurs naturally in some plants, algae, invertebrate animal species (including nematodes, some tardigrades, water fleas, some scorpions, aphids, some mites, some bees, some Phasmatodea, and parasitic wasps), and a few vertebrates, such as some fish, amphibians, reptiles,[6][7][8] and birds.[9][10][11] This type of reproduction has been induced artificially in a number of animal species that naturally reproduce through sex, including fish, amphibians, and mice.[12][13]
Some species reproduce exclusively by parthenogenesis (such as the bdelloid rotifers), while others can switch between sexual reproduction and parthenogenesis. This is called facultative parthenogenesis (other terms are cyclical parthenogenesis, heterogamy[14][15] or heterogony[16][17]). The switch between sexuality and parthenogenesis in such species may be triggered by the season (aphid, some gall wasps), or by a lack of males or by conditions that favour rapid population growth (rotifers and cladocerans like Daphnia). In these species asexual reproduction occurs either in summer (aphids) or as long as conditions are favourable. This is because in asexual reproduction a successful genotype can spread quickly without being modified by sex or wasting resources on male offspring who will not give birth. Some species can produce both sexually and through parthenogenesis, and offspring in the same clutch of a species of tropical lizard can be a mix of sexually produced offspring and parthenogenically produced offspring.[18] In California condors, facultative parthenogenesis can occur even when a male is present and available for a female to breed with.[19] In times of stress, offspring produced by sexual reproduction may be fitter as they have new, possibly beneficial gene combinations. In addition, sexual reproduction provides the benefit of meiotic recombination between non-sister chromosomes, a process associated with repair of DNA double-strand breaks and other DNA damages that may be induced by stressful conditions.[20]
Many taxa with heterogony have within them species that have lost the sexual phase and are now completely asexual. Many other cases of obligate parthenogenesis (or gynogenesis) are found among polyploids and hybrids where the chromosomes cannot pair for meiosis. [citation needed]
The production of female offspring by parthenogenesis is referred to as thelytoky (e.g., aphids) while the production of males by parthenogenesis is referred to as arrhenotoky (e.g., bees). When unfertilized eggs develop into both males and females, the phenomenon is called deuterotoky.[21]
Parthenogenesis can occur without meiosis through mitotic oogenesis. This is called apomictic parthenogenesis. Mature egg cells are produced by mitotic divisions, and these cells directly develop into embryos. In flowering plants, cells of the gametophyte can undergo this process. The offspring produced by apomictic parthenogenesis are full clones of their mother, as in aphids.[citation needed]
Parthenogenesis involving meiosis is more complicated. In some cases, the offspring are haploid (e.g., male ants). In other cases, collectively called automictic parthenogenesis, the ploidy is restored to diploidy by various means. This is because haploid individuals are not viable in most species. In automictic parthenogenesis, the offspring differ from one another and from their mother. They are called half clones of their mother. [citation needed]

Automixis includes several reproductive mechanisms, some of which are parthenogenetic.[22][23]
Diploidy can be restored by the doubling of the chromosomes without cell division before meiosis begins or after meiosis is completed. This is an endomitotic cycle. Diploidy can also be restored by fusion of the first two blastomeres, or by fusion of the meiotic products. The chromosomes may not separate at one of the two anaphases (restitutional meiosis)l; or the nuclei produced may fuse; or one of the polar bodies may fuse with the egg cell at some stage during its maturation.[citation needed]
Some authors consider all forms of automixis sexual as they involve recombination. Many others classify the endomitotic variants as asexual and consider the resulting embryos parthenogenetic. Among these authors, the threshold for classifying automixis as a sexual process depends on when the products of anaphase I or of anaphase II are joined. The criterion for sexuality varies from all cases of restitutional meiosis,[24] to those where the nuclei fuse or to only those where gametes are mature at the time of fusion.[23] Those cases of automixis that are classified as sexual reproduction are compared to self-fertilization in their mechanism and consequences.[citation needed]
The genetic composition of the offspring depends on what type of automixis takes place. When endomitosis occurs before meiosis[25][26] or when central fusion occurs (restitutional meiosis of anaphase I or the fusion of its products), the offspring get all[25][27] to more than half of the mother's genetic material and heterozygosity is mostly preserved[28] (if the mother has two alleles for a locus, it is likely that the offspring will get both). This is because in anaphase I the homologous chromosomes are separated. Heterozygosity is not completely preserved when crossing over occurs in central fusion.[29] In the case of pre-meiotic doubling, recombination, if it happens, occurs between identical sister chromatids.[25]
If terminal fusion (restitutional meiosis of anaphase II or the fusion of its products) occurs, a little over half the mother's genetic material is present in the offspring and the offspring are mostly homozygous.[30] This is because at anaphase II the sister chromatids are separated and whatever heterozygosity is present is due to crossing over. In the case of endomitosis after meiosis, the offspring is completely homozygous and has only half the mother's genetic material.[citation needed] This can result in parthenogenetic offspring being unique from each other and from their mother. [citation needed]
In apomictic parthenogenesis, the offspring are clones of the mother and hence (except for aphids) are usually female. In the case of aphids, parthenogenetically produced males and females are clones of their mother except that the males lack one of the X chromosomes (XO).[31]
When meiosis is involved, the sex of the offspring depends on the type of sex determination system and the type of apomixis. In species that use the XY sex-determination system, parthenogenetic offspring have two X chromosomes and are female. In species that use the ZW sex-determination system the offspring genotype may be one of ZW (female),[27][28] ZZ (male), or WW (non-viable in most species,[30] but a fertile,[dubious – discuss] viable female in a few, e.g., boas).[30] ZW offspring are produced by endoreplication before meiosis or by central fusion.[27][28] ZZ and WW offspring occur either by terminal fusion[30] or by endomitosis in the egg cell.[citation needed]
In polyploid obligate parthenogens, like the whiptail lizard, all the offspring are female.[26]
In many hymenopteran insects such as honeybees, female eggs are produced sexually, using sperm from a drone father, while the production of further drones (males) depends on the queen (and occasionally workers) producing unfertilized eggs. This means that females (workers and queens) are always diploid, while males (drones) are always haploid, and produced parthenogenetically.[citation needed]
Facultative parthenogenesis occurs when a female can produce offspring either sexually or via asexual reproduction.[32] Facultative parthenogenesis is extremely rare in nature, with only a few examples of animal taxa capable of facultative parthenogenesis.[32] One of the best-known examples of taxa exhibiting facultative parthenogenesis are mayflies; presumably, this is the default reproductive mode of all species in this insect order.[33] Facultative parthenogenesis has generally been believed to be a response to a lack of a viable male. A female may undergo facultative parthenogenesis if a male is absent from the habitat or if it is unable to produce viable offspring. However, California condors and the tropical lizard Lepidophyma smithii both can produce parthenogenic offspring in the presence of males, indicating that facultative parthenogenesis may be more common than previously thought and is not simply a response to a lack of males.[18][10]
In aphids, a generation sexually conceived by a male and a female produces only females. The reason for this is the non-random segregation of the sex chromosomes 'X' and 'O' during spermatogenesis.[34]
Facultative parthenogenesis is often used to describe cases of spontaneous parthenogenesis in normally sexual animals.[35] For example, many cases of spontaneous parthenogenesis in sharks, some snakes, Komodo dragons, and a variety of domesticated birds were widely attributed to facultative parthenogenesis.[36] These cases are examples of spontaneous parthenogenesis.[32][35] The occurrence of such asexually produced eggs in sexual animals can be explained by a meiotic error, leading to eggs produced via automixis.[35][37]
Obligate parthenogenesis is the process in which organisms exclusively reproduce through asexual means.[38] Many species have transitioned to obligate parthenogenesis over evolutionary time. Well documented transitions to obligate parthenogenesis have been found in numerous metazoan taxa, albeit through highly diverse mechanisms. These transitions often occur as a result of inbreeding or mutation within large populations.[39] Some documented species, specifically salamanders and geckos, that rely on obligate parthenogenesis as their major method of reproduction. As such, there are over 80 species of unisex reptiles (mostly lizards but including a single snake species), amphibians and fishes in nature for which males are no longer a part of the reproductive process.[40] A female produces an ovum with a full set (two sets of genes) provided solely by the mother. Thus, a male is not needed to provide sperm to fertilize the egg. This form of asexual reproduction is thought in some cases to be a serious threat to biodiversity for the subsequent lack of gene variation and potentially decreased fitness of the offspring.[38]
Some invertebrate species that feature (partial) sexual reproduction in their native range are found to reproduce solely by parthenogenesis in areas to which they have been introduced.[41][42] Relying solely on parthenogenetic reproduction has several advantages for an invasive species: it obviates the need for individuals in a very sparse initial population to search for mates; and an exclusively female sex distribution allows a population to multiply and invade more rapidly (potentially twice as fast). Examples include several aphid species[41] and the willow sawfly, Nematus oligospilus, which is sexual in its native Holarctic habitat but parthenogenetic where it has been introduced into the Southern Hemisphere.[42]
Parthenogenesis does not apply to isogamous species.[43] Parthenogenesis occurs naturally in aphids, Daphnia, rotifers, nematodes, and some other invertebrates, as well as in many plants. Among vertebrates, strict parthenogenesis is only known to occur in lizards, snakes,[44] birds,[45] and sharks.[46] Fish, amphibians, and reptiles make use of various forms of gynogenesis and hybridogenesis (an incomplete form of parthenogenesis).[47] The first all-female (unisexual) reproduction in vertebrates was described in the fish Poecilia formosa in 1932.[48] Since then at least 50 species of unisexual vertebrate have been described, including at least 20 fish, 25 lizards, a single snake species, frogs, and salamanders.[47]
Use of an electrical or chemical stimulus can produce the beginning of the process of parthenogenesis in the asexual development of viable offspring.[49]

During oocyte development, high metaphase promoting factor (MPF) activity causes mammalian oocytes to arrest at the metaphase II stage until fertilization by a sperm. The fertilization event causes intracellular calcium oscillations, and targeted degradation of cyclin B, a regulatory subunit of MPF, thus permitting the MII-arrested oocyte to proceed through meiosis.[50][51] To initiate parthenogenesis of swine oocytes, various methods exist to induce an artificial activation that mimics sperm entry, such as calcium ionophore treatment, microinjection of calcium ions, or electrical stimulation. Treatment with cycloheximide, a non-specific protein synthesis inhibitor, enhances parthenote development in swine presumably by continual inhibition of MPF/cyclin B.[51] As meiosis proceeds, extrusion of the second polar is blocked by exposure to cytochalasin B. This treatment results in a diploid (2 maternal genomes) parthenote[50] Parthenotes can be surgically transferred to a recipient oviduct for further development, but will succumb to developmental failure after ≈30 days of gestation. The swine parthenote placentae often appears hypo-vascular: see free image (Figure 1) in linked reference.[50]
Induced parthenogenesis in mice and monkeys often results in abnormal development. This is because mammals have imprinted genetic regions, where either the maternal or the paternal chromosome is inactivated in the offspring for development to proceed normally. A mammal created by parthenogenesis would have double doses of maternally imprinted genes and lack paternally imprinted genes, leading to developmental abnormalities. It has been suggested that defects in placental folding or interdigitation are one cause of swine parthenote abortive development.[50] As a consequence, research on human parthenogenesis is focused on the production of embryonic stem cells for use in medical treatment, not as a reproductive strategy. In 2022, researchers reported that they have achieved parthenogenesis in mice for viable offspring born from unfertilized eggs, addressing the problems of genomic imprinting by "targeted DNA methylation rewriting of seven imprinting control regions".[13]
In 1955, Helen Spurway, a geneticist specializing in the reproductive biology of the guppy (Lebistes reticulatus), claimed that parthenogenesis may occur (though very rarely) in humans, leading to so-called "virgin births". This created some sensation among her colleagues and the lay public alike.[52] Sometimes an embryo may begin to divide without fertilization, but it cannot fully develop on its own; so while it may create some skin and nerve cells, it cannot create others (such as skeletal muscle) and becomes a type of benign tumor called an ovarian teratoma.[53] Spontaneous ovarian activation is not rare and has been known about since the 19th century. Some teratomas can even become primitive fetuses (fetiform teratoma) with imperfect heads, limbs and other structures, but are non-viable.[citation needed]
In 1995, there was a reported case of partial human parthenogenesis; a boy was found to have some of his cells (such as white blood cells) to be lacking in any genetic content from his father. Scientists believe that an unfertilized egg began to self-divide but then had some (but not all) of its cells fertilized by a sperm cell; this must have happened early in development, as self-activated eggs quickly lose their ability to be fertilized. The unfertilized cells eventually duplicated their DNA, boosting their chromosomes to 46. When the unfertilized cells hit a developmental block, the fertilized cells took over and developed that tissue. The boy had asymmetrical facial features and learning difficulties but was otherwise healthy. This would make him a parthenogenetic chimera (a child with two cell lineages in his body).[54] While over a dozen similar cases have been reported since then (usually discovered after the patient demonstrated clinical abnormalities), there have been no scientifically confirmed reports of a non-chimeric, clinically healthy human parthenote (i.e. produced from a single, parthenogenetic-activated oocyte).[53]
In 2007, the International Stem Cell Corporation of California announced that Elena Revazova had intentionally created human stem cells from unfertilized human eggs using parthenogenesis. The process may offer a way for creating stem cells genetically matched to a particular female to treat degenerative diseases. The same year, Revazova and ISCC published an article describing how to produce human stem cells that are homozygous in the HLA region of DNA.[55] These stem cells are called HLA homozygous parthenogenetic human stem cells (hpSC-Hhom) and would allow derivatives of these cells to be implanted without immune rejection. With selection of oocyte donors according to HLA haplotype, it would be possible to generate a bank of cell lines whose tissue derivatives, collectively, could be MHC-matched with a significant number of individuals within the human population.[56]
After an independent investigation, it was revealed that the discredited South Korean scientist Hwang Woo-Suk unknowingly produced the first human embryos resulting from parthenogenesis. Initially, Hwang claimed he and his team had extracted stem cells from cloned human embryos, a result later found to be fabricated. Further examination of the chromosomes of these cells show indicators of parthenogenesis in those extracted stem cells, similar to those found in the mice created by Tokyo scientists in 2004. Although Hwang deceived the world about being the first to create artificially cloned human embryos, he contributed a major breakthrough to stem cell research by creating human embryos using parthenogenesis.[57]
A form of asexual reproduction related to parthenogenesis is gynogenesis. Here, offspring are produced by the same mechanism as in parthenogenesis, but with the requirement that the egg merely be stimulated by the presence of sperm in order to develop. However, the sperm cell does not contribute any genetic material to the offspring. Since gynogenetic species are all female, activation of their eggs requires mating with males of a closely related species for the needed stimulus. Some salamanders of the genus Ambystoma are gynogenetic and appear to have been so for over a million years. The success of those salamanders may be due to rare fertilization of eggs by males, introducing new material to the gene pool, which may result from perhaps only one mating out of a million. In addition, the amazon molly is known to reproduce by gynogenesis.[58]
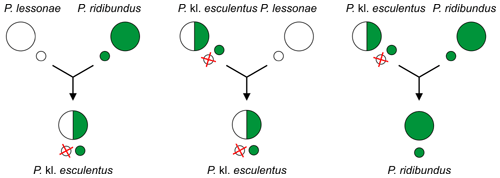
Hybridogenesis is a mode of reproduction of hybrids. Hybridogenetic hybrids (for example AB genome), usually females, during gametogenesis exclude one of parental genomes (A) and produce gametes with unrecombined[59] genome of second parental species (B), instead of containing mixed recombined parental genomes. First genome (A) is restored by fertilization of these gametes with gametes from the first species (AA, sexual host,[59] usually male).[59][61][62] Hybridogenesis is not completely asexual, but hemiclonal: half the genome is passed to the next generation clonally, unrecombined, intact (B), other half sexually, recombined (A). This process continues, so that each generation is half (or hemi-) clonal on the mother's side and has half new genetic material from the father's side.[59][63]
This form of reproduction is seen in some live-bearing fish of the genus Poeciliopsis[61][64] as well as in some of the Pelophylax spp. ("green frogs" or "waterfrogs"):
Other examples where hybridogenesis is at least one of modes of reproduction include i.e.
Parthenogenesis, in the form of reproduction from a single individual (typically a god), is common in mythology, religion, and folklore around the world, including in ancient Greek myth; for example, Athena was born from the head of Zeus.[70] In Christianity and Islam, there is the virgin birth of Jesus; there are stories of miraculous births in other religions including Islam.[71] The theme is one of several aspects of reproductive biology explored in science fiction.[72]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.