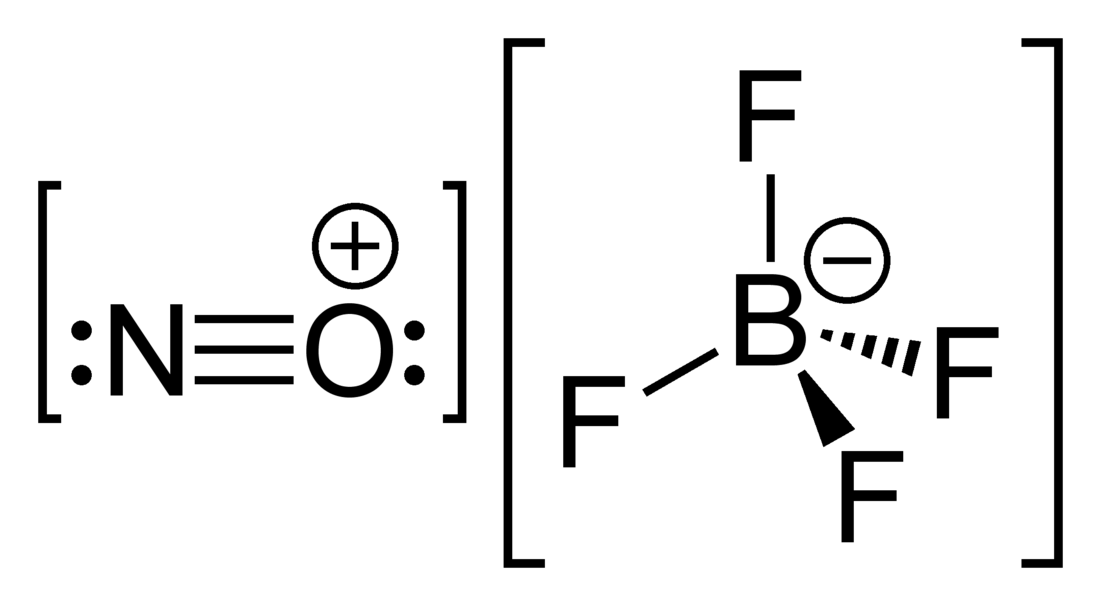Nitrosonium tetrafluoroborate
Chemical compound From Wikipedia, the free encyclopedia
Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent, diazotizing agent and a mild oxidant.[1]
 | |
| Names | |
|---|---|
| IUPAC name
nitrosonium tetrafluoroborate | |
| Other names
nitrosyl tetrafluoroborate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.148 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BF4NO | |
| Molar mass | 116.81 g·mol−1 |
| Appearance | colourless crystalline solid |
| Density | 2.185 g cm−3 |
| Melting point | 250 °C (482 °F; 523 K) (sublimes) |
| decomposes | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, [NO]+, and a tetrafluoroborate anion, [BF4]−.[2]
Reactions
The dominant property of NOBF4 is the oxidizing power and electrophilic character of the nitrosonium cation. It forms colored charge transfer complexes with hexamethylbenzene and with 18-crown-6. The latter, a deep yellow species, provides a means to dissolve NOBF4 in dichloromethane.[3]
Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type [MII(CH3CN)x][BF4]2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas:[4]
- M + 2NOBF4 + xCH3CN → [M(CH3CN)x](BF4)2 + 2NO
With ferrocene the ferrocenium tetrafluoroborate is formed.[5]
In its infrared spectrum of this salt, νNO is a strong peak at 2387 cm−1.[6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.