Apimostinel
Investigational antidepressant compound From Wikipedia, the free encyclopedia
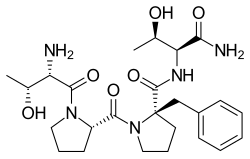
Apimostinel (GATE-202, formerly NRX-1074) is an investigational antidepressant, acting as a novel and selective modulator of the NMDA receptor.[1][2][3][4] It is currently under development for the acute treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Naurex and Allergan.[5][6][7] As of February 2015, an intravenous formulation of apimostinel has completed a phase IIa clinical trial for MDD.[5][8]
 | |
| Clinical data | |
|---|---|
| Other names | NRX-1074; AGN-241660; Threonyl-prolyl-2R-(2-benzyl)-prolyl-threonine amide |
| Routes of administration | By mouth |
| Drug class | NMDA receptor modulator |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H37N5O6 |
| Molar mass | 503.600 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Similar to rapastinel (GLYX-13), its mechanism of action acts through a unique binding site on the NMDA receptor, independent of the glycine site, to modulate receptor activity and enhance NMDAR-mediated synaptic plasticity.[9] However, apimostinel is 1000-fold more potent in vitro and is intended as an improved, follow-up drug to rapastinel.[2][5] Similar to rapastinel, apimostinel is an amidated tetrapeptide, but has been structurally modified, via the addition of a benzyl group, to enhance its metabolic stability and pharmacokinetic profile. The drug has shown rapid and potent antidepressant effects in pre-clinical models of depression.[5] In addition, similarly to rapastinel, it is well tolerated and lacks the schizophrenia-like psychotomimetic effects of NMDA receptor antagonists such as ketamine.[5]
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
