Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.
 | |
| Names | |
|---|---|
| IUPAC name
Molybdenum tetrachloride | |
| Other names
Molybdenum(IV) chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.039 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| MoCl4 | |
| Molar mass | 237.752 g/mol |
| Appearance | black solid |
| Melting point | 552 °C (1,026 °F; 825 K) |
| Decomposes | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non flammable |
| Related compounds | |
Related compounds |
Molybdenum(II) chloride Molybdenum(III) chloride Molybdenum(V) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
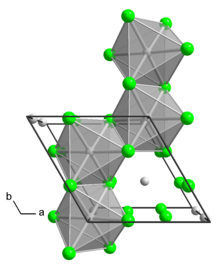
The α polymorph is a polymer. The β polymorph is a hexamer. In both polymorph, the Mo center is octahedral with two terminal chloride ligands and four doubly bridging ligands.[1] In addition to these two binary phases, a number of adducts are know with the formula MoCl4L2 where L is a Lewis base.
α-Molybdenum tetrachloride can be prepared from by dechlorination of molybdenum pentachloride using tetrachloroethene:[2]
Heating α-molybdenum tetrachloride in a sealed container in the presence of molybdenum pentachloride induces conversion to the β polymorph.[2]
When heated in an open container, molybdenum tetrachloride evolves chlorine, giving molybdenum trichloride;[2]
The acetonitrile complex adduct can be prepared by reduction of the pentachloride with acetonitrile:[3][4]
The MeCN ligands can be exchanged with other ligands:
The pentachloride can be reduced to the ether complex MoCl4(Et2O)2 using tin powder. It is a beige, paramagnetic solid.[5]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.