Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
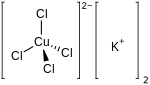
Potassium tetrachloridocuprate(II) is a salt with chemical formula K
2CuCl
4, also written as (K+
)2·[CuCl
4]2−.
 | |
| Names | |
|---|---|
| IUPAC name
Potassium tetrachloridocuprate(II) | |
| Other names
Potassium tetrachlorocuprate, potassium copper(II) tetrachloride, dipotassium cupric chloride, mitscherlichite (dihydrate mineral) | |
| Identifiers | |
| |
PubChem CID |
|
| |
| Properties | |
| K2CuCl4 (anhydrous) K2CuCl4·2H2O (dihydrate) | |
| Molar mass | 319.585 g/mol (dihydrate) |
| Appearance | greenish blue crystals (dihydrate) |
| Density | 2.416 g/cm3 at 25 °C (dihydrate)[1] |
| Structure | |
| (dihydrate:) Tetragonal.Point Group: 4/m 2/m 2/m (probable). Crystals, short prismatic along [001], or pyramidal {011}, minute; in stalactitic growths[2] | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Cesium tetrachloridocuprate(II) ammonium tetrachloridocuprate(II) rubidium tetrachloridocuprate(II) iron(II) tetrachloridocuprate(II) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The compound is often found as the dihydrate K
2CuCl
4·2H
2O, which is a brilliant greenish blue crystalline solid.[1] This form also occurs naturally as the rare mineral mitscherlichite.[1][2]
The compound is also called potassium tetrachlorocuprate(II), dipotassium tetrachlorocuprate, potassium copper(II) tetrachloride, potassium cupric chloride and other similar names.

The dihydrate occurs rarely in nature near volcanic vents, e.g. in Mount Vesuvius, as the mineral mitscherlichite; which is named in honor of Eilhardt Mitscherlich (1794–1863), the German crystallographer and chemist who first synthesized the compound.[2] It was identified as pigment in some ancient artifacts.[3]
The dihydrate can be obtained by slow evaporation of a solution of potassium chloride (KCl) and copper(II) chloride (CuCl
2) in 2:1 molar ratio.[1][4]

The crystal structure of the dihydrate was partially determined in 1927 by Hendricks and Dickinson[1][4] and refined in 1934 by Chrobak.[5] The structure is tetragonal P42/mnm (136), Z=2, isostructural with ammonium tetrachoridocuprate(II) (NH
4)2CuCl
4·2H
2O and rubidium tetrachoridocuprate(II) Rb
2CuCl
4·2H
2O.[1][4][5][6][7] Each copper atom is immediately surrounded by two oxygen atoms and four chlorine atoms forming a hydrated tetrachloridocuprate(II) anion. Two of the chlorine atoms are about 0.75 angstroms further away than the other two. Each potassium atom is surrounded by four oxygen atoms, four copper atoms and eight chlorine atoms.[4]
The anhydrous compound was reported in 1952 by C. M. Fontana and others.[8] In the next two decades others reported its heat of formation[9] and its structure.[6][7]
In the mid-1970s, however, its existence was questioned.[10][11] The phase diagram for the anhydrous system KCl/CuCl
2 shows potassium trichloridocuprate KCuCl
3 as a congruently-melting compound, but not K
2CuCl
4.[12] The dihydrate decomposes on heating above 93 °C to KCl, KCuCl
3 and water.[13][14]
The doubts were put to rest when successful dehydration was achieved by T. J. Nolan and others in 1975.[15][16]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.