Iron(II) acetate
Chemical compound From Wikipedia, the free encyclopedia
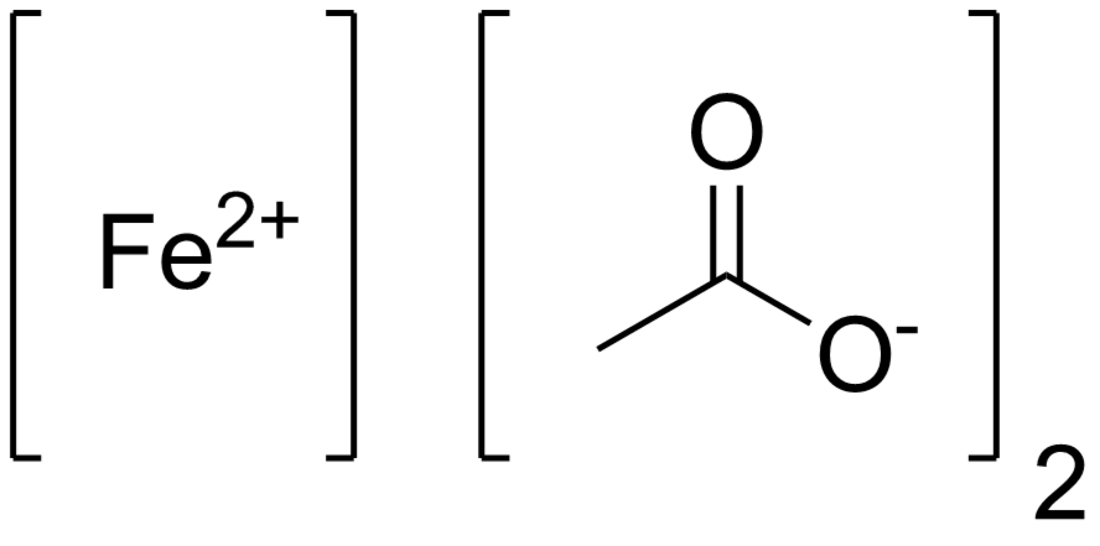
Iron(II) acetate describes compounds with formula Fe(CH3CO2)2·(H2O)x where x can be 0 (anhydrous) or 4 (tetrahydrate). The anhydrous compound is a white solid, although impure samples can be slightly colored.[1] The tetrahydrate is light green solid that is highly soluble in water.
 | |
| Names | |
|---|---|
| IUPAC name
Iron(II) acetate | |
| Other names
Ferrous acetate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.492 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6FeO4 | |
| Molar mass | 173.933 g·mol−1 |
| Appearance | White crystals (anhydrous) Light green crystals (tetrahydrate) |
| Odor | Odorless |
| Density | 1.734 g/cm3 (−73 °C)[1] |
| Melting point | 190–200 °C (374–392 °F; 463–473 K) decomposes[2][3] |
| Soluble[2] | |
| Structure | |
| Orthorhombic, oP75 (200 K) | |
| Pbcn, No. 60 (200 K)[1] | |
| 2/m 2/m 2/m (200 K) | |
a = 18.1715(4) Å, b = 22.1453(5) Å, c = 8.2781(2) Å (200 K) α = 90°, β = 90°, γ = 90° | |
| Hazards | |
| GHS labelling: | |
 [3] [3] | |
| Warning | |
| H315, H319, H335[3] | |
| P261, P305+P351+P338[3] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation and structure

Iron powder reacts with acetic acid to give the ferrous acetate, with evolution of hydrogen gas:[5][1]
- Fe + 2 CH3CO2H → Fe(CH3CO2)2 + H2
Reaction of scrap iron with acetic acid affords a brown mixture of various iron(II) and iron(III) acetates that are used in dyeing.[6]
It can also be made from the insoluble, olive green, Iron(II) carbonate.[citation needed]
It adopts a polymeric structure with octahedral Fe(II) centers interconnected by acetate ligands. It is a coordination polymer.[1]
Uses
Ferrous acetate is used as a mordant by the dye industry. Ebonizing wood is one such process.[7]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.