Top Qs
Timeline
Chat
Perspective
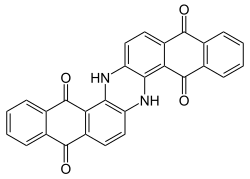
Indanthrone blue
Organic dye made from 2-aminoanthraquinone From Wikipedia, the free encyclopedia
Remove ads
Indanthrone blue, also called indanthrene, is an organic compound with the formula (C14H6O2NH)2. It is a dark blue solid that is a common dye as well as a precursor to other dyes.[1]
Remove ads
Preparation
The compound is made from 2-aminoanthraquinone treated with potassium hydroxide.
By dimerization of 2-aminoanthraquinone (1) under strongly alkaline conditions at 220-235 °C, the intermediate 3 is obtained in two steps, which cyclizes intramolecularly and is oxidized to indanthrone 5.[2]
Applications
Summarize
Perspective
It is a pigment that can be used in the following media: acrylic, alkyd, casein, encaustic, fresco, gouache, linseed oil, tempera, pastel, and watercolor painting. It is used to dye unmordanted cotton and as a pigment in quality paints and enamels. As a food dye, it has number 130, but it is not approved for use in either the United States or the European Union.[3][4] It has excellent light fastness, but may bleed in some organic solvents.
Organic Semiconductor
As an organic semiconductor, indanthrone has capabilities for utilization as a photocatalyst for oxygen generation from water utilizing solar energy.[5][6] Indanthrone's characteristic as a nonlinear optics light absorber, allows for its use as an optical limiter, which can be employed, for instance, in laser protective filters.[7]
Colorant
Indanthrone is utilized as a blue pigment (C.I. Pigment Blue 60), primarily in the process of vat dyeing, often referred to as C.I. Vat Blue 4.[8] Indanthrone is a vat dye, synthesized to provide highest color fastness for the dyeing and printing of predominantly cellulose-based textile fibers. Fabrics dyed with indanthrene fulfill the highest standards and exhibit exceptional wash fastness, boil fastness, light fastness, weather fastness and chlorine fastness.
Trademark
Indanthrone blue was the first example of the brand "Indanthren" (an acronym for Indigo from anthracene) introduced by BASF in 1901.[9][10][11] One result is that even now, in Japan vat dyes are commonly described as threne dyes (スレン染料), derived from the Japanese transliteration of the brand.[12][13]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads