Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Hydrogen peroxide–urea (also called Hyperol, artizone, urea hydrogen peroxide, and UHP) is a white crystalline solid chemical compound composed of equal amounts of hydrogen peroxide and urea. It contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in dentistry, it is used as a source of hydrogen peroxide when dissolved in water for bleaching, disinfection and oxidation.
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hydrogen peroxide–urea (1/1) | |||
| Systematic IUPAC name
Peroxol–carbonyl diamide (1/1) | |||
| Other names
Urea peroxide, percarbamide, UHP | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.275 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH6N2O3 | |||
| Molar mass | 94.070 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.50 g/cm3 | ||
| Melting point | 75 to 91.5 °C (167.0 to 196.7 °F; 348.1 to 364.6 K) (decomposes) | ||
| Pharmacology | |||
| D02AE01 (WHO) | |||
| Hazards | |||
| GHS labelling:[1] | |||
  | |||
| Danger | |||
| H272, H315, H318 | |||
| P210, P220, P264, P280, P302+P352, P305+P351+P338 | |||
| Flash point | 60 °C (140 °F; 333 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
For the preparation of the complex, urea is dissolved in 30% hydrogen peroxide (molar ratio 2:3) at temperatures below 60 °C. upon cooling this solution, hydrogen peroxide–urea precipitates in the form of small platelets.[2]
Akin to water of crystallization, hydrogen peroxide cocrystallizes with urea with the stoichiometry of 1:1. The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization.[3] The laboratory synthesis is analogous.[4]
The solid state structure of this adduct has been determined by neutron diffraction.[5]
Hydrogen peroxide–urea is a readily water-soluble, odorless, crystalline solid, which is available as white powder or colorless needles or platelets.[2] Upon dissolving in various solvents, the 1:1 complex dissociates back to urea and hydrogen peroxide. So just like hydrogen peroxide, the (erroneously) so-called adduct is an oxidizer but the release at room temperature in the presence of catalysts proceeds in a controlled manner. Thus the compound is suitable as a safe substitute for the unstable aqueous solution of hydrogen peroxide. Because of the tendency for thermal decomposition, which accelerates at temperatures above 82 °C,[6] it should not be heated above 60 °C, particularly in pure form.
The solubility of commercial samples varies from 0.05 g/mL[7] to more than 0.6 g/mL.[8]
Hydrogen peroxide–urea is mainly used as a disinfecting and bleaching agent in cosmetics and pharmaceuticals.[3] As a drug, this compound is used in some preparations for the whitening of teeth.[3][9][10] It is also used to relieve minor inflammation of gums, oral mucosal surfaces and lips including canker sores and dental irritation,[11] and to emulsify and disperse earwax.[12]
Carbamide peroxide is also suitable as a disinfectant, e.g. for germ reduction on contact lens surfaces or as an antiseptic for mouthwashes, ear drops or for superficial wounds and ulcers.
In the laboratory, it is used as a more easily handled replacement for hydrogen peroxide.[4][13][14] It has proven to be a stable, easy-to-handle and effective oxidizing agent which is readily controllable by a suitable choice of the reaction conditions. It delivers oxidation products in an environmentally friendly manner and often in high yields especially in the presence of organic catalysts such as cis-butenedioic anhydride[15] or inorganic catalysts such as sodium tungstate.[16]

It converts thiols selectively to disulfides,[15] secondary alcohols to ketones,[16] sulfides to sulfoxides and sulfones,[17] nitriles to amides,[17][18] and N-heterocycles to amine oxides.[17][19]

Hydroxybenzaldehydes are converted to dihydroxybenzenes (Dakin reaction)[17][20] and give, under suitable conditions, the corresponding benzoic acids.[20]

It oxidizes ketones to esters, in particular cyclic ketones, such as substituted cyclohexanones[21] or cyclobutanones[22] to give lactones (Baeyer–Villiger oxidation).
The epoxidation of various alkenes in the presence of benzonitrile yields oxiranes in yields of 79 to 96%.[23]
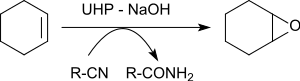
The oxygen atom transferred to the alkene originates from the peroxoimide acid formed intermediately from benzonitrile. The resulting imidic acid tautomerizes to the benzamide.
The compound acts as a strong oxidizing agent and can cause skin irritation and severe eye damage.[24] Urea–hydrogen peroxide was also found to be an insensitive high explosive, capable of detonation by strong impulse under heavy confinement.[25][26]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.