Top Qs
Timeline
Chat
Perspective
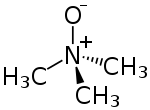
Trimethylamine N-oxide
TMAO Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Trimethylamine N-oxide (TMAO) is an organic compound with the formula (CH3)3NO. It is in the class of amine oxides. Although the anhydrous compound is known, trimethylamine N-oxide is usually encountered as the dihydrate. Both the anhydrous and hydrated materials are white, water-soluble solids.
TMAO is found in the tissues of marine crustaceans and marine fish, where it prevents water pressure from distorting proteins and thus killing the animal. The concentration of TMAO increases with the depth at which the animal lives; TMAO is found in high concentrations in the deepest-living described fish species, Pseudoliparis swirei, which was found in the Mariana Trench, at a recorded depth of 8,076 m (26,496 ft).[1][2]
In animals, TMAO is a product of the oxidation of trimethylamine, a common metabolite of trimethyl quaternary ammonium compounds, like choline, trimethylglycine, and L-carnitine.[3] High TMAO concentrations are associated with an increased risk of all-cause mortality and cardiovascular disease.[4][5][6]
Remove ads
Marine animals
Trimethylamine N-oxide is an osmolyte found in molluscs, crustaceans, and all marine fishes and bony fishes. It is a protein stabilizer that serves to counteract the protein-destabilizing effects of pressure. In general, the bodies of animals living at great depths are adapted to high pressure environments by having pressure-resistant biomolecules and small organic molecules present in their cells, known as piezolytes, of which TMAO is the most abundant. These piezolytes give the proteins the flexibility they need to function properly under great pressure.[1][2][7][8][9]
TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.[citation needed]
Remove ads
TMAO in diet
TMAO levels increase with consumption of animal protein such as eggs, red meat, shellfish and total fish consumption.[10][11] Plant-based diets such as vegan, vegetarian and the Mediterranean diet lower TMAO levels.[11][12]
Chemistry
Summarize
Perspective
TMAO can be synthesized from trimethylamine by treatment with hydrogen peroxide:[13]
- (CH3)3N + H2O2 → H2O + (CH3)3NO
The dihydrate is dehydrated by azeotropic distillation from dimethylformamide.[14]
Laboratory applications
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.[15]
In the organometallic chemistry reaction of nucleophilic abstraction, (CH3)3NO is employed as a decarbonylation agent according to the following stoichiometry:
where M is a metal. This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.[13]
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the corresponding aldehyde.[16]
Effects on protein stability
The effects of TMAO on the backbone and charged residues of peptides are found to stabilize compact conformations,[17] whereas effects of TMAO on nonpolar residues lead to peptide swelling. This suggests competing mechanisms of TMAO on proteins, which accounts for hydrophobic swelling, backbone collapse, and stabilization of charge-charge interactions. These mechanisms are observed in Trp cage.[18]
Disorders
Trimethylaminuria
Trimethylaminuria is a rare defect in the production of the enzyme flavin-containing monooxygenase 3 (FMO3).[19][20] Those suffering from trimethylaminuria are unable to convert choline-derived trimethylamine into trimethylamine oxide. Trimethylamine then accumulates and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.[citation needed]
Remove ads
Health effects
Mortality
High circulating TMAO concentrations are associated with an increased risk of all-cause mortality.[4][21]
Cardiovascular disease
High circulating TMAO concentrations are associated with an increased risk of cardiovascular events[4][21] and strokes in particular.[22]
Hypertension
High circulating TMAO concentrations are associated with an increased risk of hypertension.[23][24]
Potential toxicity
Exposure limit guidelines with a detailed description of toxicity are available such as "Recommendation from the Scientific Committee on Occupational Exposure Limits" by the European Union Commission.[25]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads