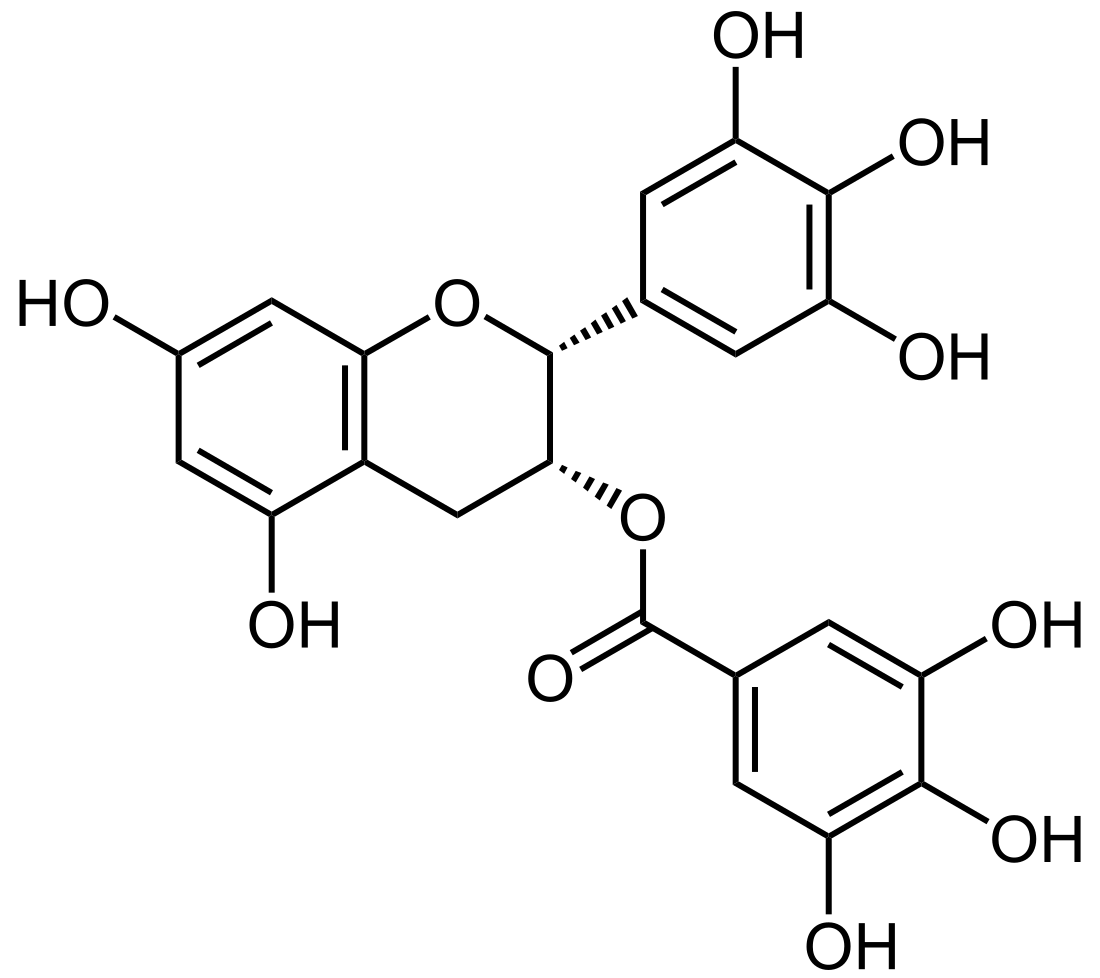
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-3′,4′,5,5′,7-Pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate | |
| Systematic IUPAC name
(2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate | |
| Other names
(-)-Epigallocatechin gallate (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl gallate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.111.017 |
| KEGG | |
| MeSH | Epigallocatechin+gallate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H18O11 | |
| Molar mass | 458.372 g/mol |
| soluble (5 g/L)[vague][1] | |
| Solubility | soluble in ethanol, DMSO, dimethyl formamide[1] at about 20 g/L |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
EGCG – the most abundant catechin in tea – is a polyphenol under basic research for its potential to affect human health and disease. EGCG is used in many dietary supplements.
Food sources
Tea
It is found in high content in the dried leaves of green tea (7380 mg per 100 g), white tea (4245 mg per 100 g), and in smaller quantities, black tea (936 mg per 100 g).[2] During black tea production, the catechins are mostly converted to theaflavins and thearubigins via polyphenol oxidases.[which?][3]
Other
Trace amounts are found in apple skin, plums, onions, hazelnuts, pecans, and carob powder (at 109 mg per 100 g).[2]
Bioavailability
When taken orally, EGCG has poor absorption even at daily intake equivalent to 8–16 cups of green tea, an amount causing adverse effects such as nausea or heartburn.[4] After consumption, EGCG blood levels peak within 1.7 hours.[5] The absorbed plasma half-life is ~5 hours,[5] but with majority of unchanged EGCG excreted into urine over 0 to 8 hours.[5] Methylated metabolites appear to have longer half-lives and occur at 8–25 times the plasma levels of unmetabolized EGCG.[6]
Research
Well-studied in basic research, EGCG has various biological effects in laboratory studies.[7][8][9][10]
A 2011 analysis by the European Food Safety Authority found that a cause and effect relationship could not be shown for a link between tea catechins and the maintenance of normal blood LDL-cholesterol concentration.[11] A 2016 review found that high daily doses (107 to 856 mg/day) taken by human subjects over four to 14 weeks produced a small reduction of LDL cholesterol.[12]
Potential toxicity
A 2018 review showed that excessive intake of EGCG may cause liver toxicity.[13] In 2018, the European Food Safety Authority stated that daily intake of 800 mg or more could increase risk of liver damage.[14]
Taken as a capsule or tablet 338 mg per day of EGCG is considered safe, whereas 704 mg per day is safe if consumed as a tea beverage.[13] 100 mL of green tea contains about 70.2 mg of EGCG (about 165 mg per cup).[14]
Regulation
Over 2008 to 2017, the US Food and Drug Administration issued several warning letters to manufacturers of dietary supplements containing EGCG for violations of the Federal Food, Drug, and Cosmetic Act. Most of these letters informed the companies that their promotional materials promoted EGCG-based dietary supplements in the treatment or prevention of diseases or conditions that cause them to be classified as drugs under the United States code,[15][16][17] while another focused on inadequate quality assurance procedures and labeling violations.[18] The warnings were issued because the products had not been established as safe and effective for their marketed uses and were promoted as "new drugs", without approval as required under the Act.[17]
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.