Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
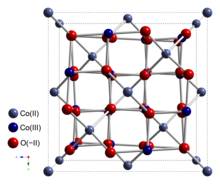
Cobalt(II,III) oxide is an inorganic compound with the formula Co3O4. It is one of two well characterized cobalt oxides. It is a black antiferromagnetic solid. As a mixed valence compound, its formula is sometimes written as CoIICoIII2O4 and sometimes as CoO•Co2O3.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
cobalt(II) dicobalt(III) oxide | |
| Other names
cobalt oxide, cobalt(II,III) oxide, cobaltosic oxide, tricobalt tetroxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.780 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Co3O4 CoO.Co2O3 | |
| Molar mass | 240.80 g/mol |
| Appearance | black solid |
| Density | 6.07 g/cm3[2] |
| Melting point | 895 °C (1,643 °F; 1,168 K) |
| Boiling point | 900 °C (1,650 °F; 1,170 K) (decomposes) |
| Insoluble | |
| Solubility | soluble (with degradation) in acids and alkalis |
| +7380·10−6 cm3/mol | |
| Structure | |
| cubic | |
| Fd3m, No. 227[3] | |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H317, H334, H350, H411 | |
| P261, P273, P284, P304+P340, P342+P311 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Co3O4 adopts the normal spinel structure, with Co2+ ions in tetrahedral interstices and Co3+ ions in the octahedral interstices of the cubic close-packed lattice of oxide anions.[4]
 | 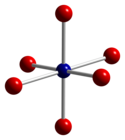 | 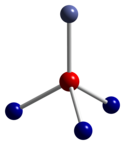 |
| tetrahedral coordination geometry of Co(II) | distorted octahedral coordination geometry of Co(III) | distorted tetrahedral coordination geometry of O |
Cobalt(II) oxide, CoO, converts to Co3O4 upon heating at around 600–700 °C in air.[4] Above 900 °C, CoO is stable.[4][5] These reactions are described by the following equilibrium:
Cobalt(II,III) oxide is used as a blue coloring agent for pottery enamel and glass, as an alternative to cobalt(II) oxide.[6]
Cobalt(II,III) oxide is used as an electrode in some lithium-ion batteries, possibly in the form of cobalt oxide nanoparticles.
Cobalt compounds are potentially poisonous in large amounts.[7]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.