Methylchloroisothiazolinone
Chemical compound From Wikipedia, the free encyclopedia
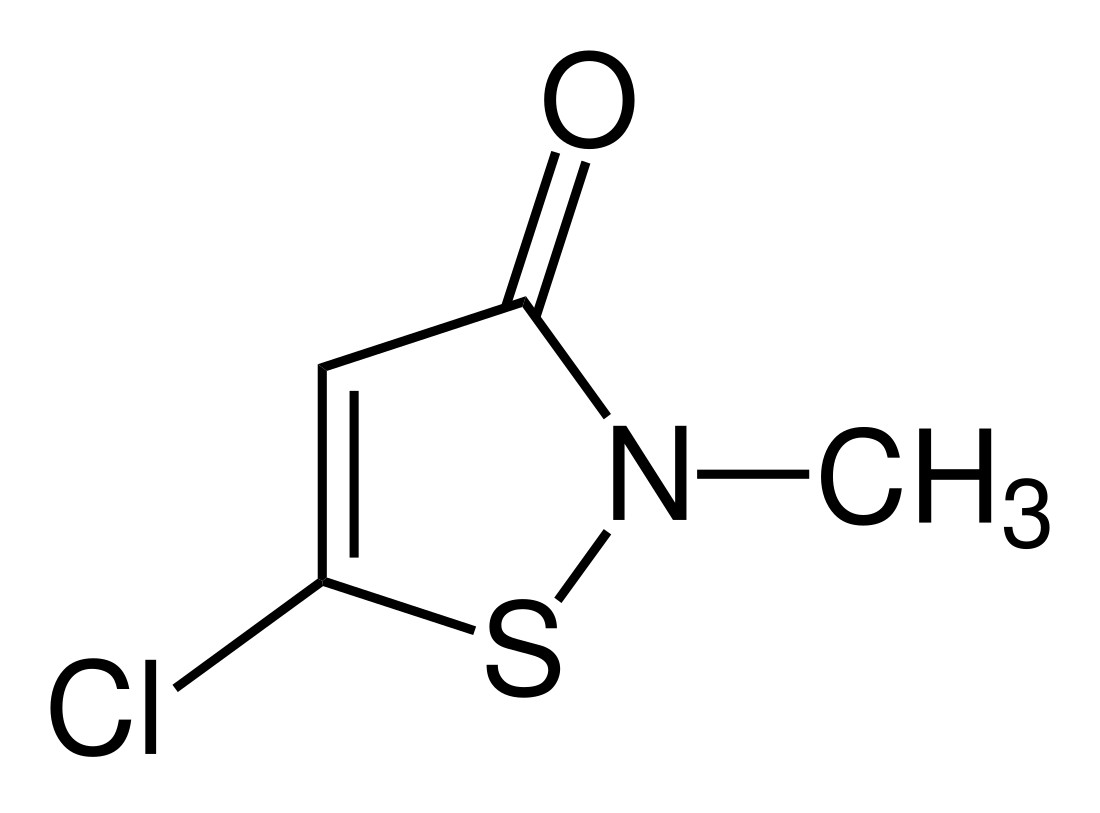
Methylchloroisothiazolinone, also referred to as MCI, is the organic compound with the formula S(C2HCl)C(O)N(CH3). It is a white solid that melts near room temperature. The compound is an isothiazolinone, a class of heterocycles used as biocides. These compounds have an active sulphur moiety that is able to oxidize thiol-containing residues, thereby effectively killing most aerobic and anaerobic bacteria. MCI is often used in combination with methylisothiazolinone, a mixture known as Kathon. The isothiazolinones have attracted attention because they can cause contact dermatitis.[1][2][3] Methylchloroisothiazolinone is effective against gram-positive and gram-negative bacteria, yeast, and fungi.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
5-Chloro-2-methyl-1,2-thiazol-3(2H)-one | |
| Other names
5-Chloro-2-methylisothiazol-3(2H)-one 5-Chloro-2-methyl-4-isothiazolin-3-one Chloromethylisothiazolinone Chloromethylisothiazolone Methylchloroisothiazolinone Methylchloroisothiazolone CMI CMIT MCI MCIT CIT | |
| Identifiers | |
3D model (JSmol) |
|
| 1210149 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.043.167 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4ClNOS | |
| Molar mass | 149.59 g·mol−1 |
| Appearance | white solid |
| Density | 1.02 g/cm3 |
| Melting point | 52 °C (126 °F; 325 K) |
| Miscible | |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H300, H301, H310, H311, H314, H317, H330, H331, H335, H410 | |
| P260, P261, P262, P264, P270, P271, P272, P273, P280, P284, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P333+P313, P361, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Application
Methylchloroisothiazolinone is found in many water-based personal care products and cosmetics.[2] Methylchloroisothiazolinone was first used in cosmetics in the 1970s. It is also used in glue production, detergents, paints, fuels, and other industrial processes. Methylchloroisothiazolinone is known by the registered tradename Kathon CG when used in combination with methylisothiazolinone.[3]
Methylchloroisothiazolinone may be used in combination with other preservatives including ethylparaben, benzalkonium chloride, bronopol and phenoxyethanol.
Hazards
Summarize
Perspective
Methylchloroisothiazolinone can cause allergic reactions in some people.[4] The first publication of the preservative as a contact allergen was in 1988.[5] Cases of photoaggravated allergic contact dermatitis, i.e. worsening of skin lesions after sun exposure, have also been reported.[4]
In pure form or in high concentrations, methylchloroisothiazolinone is a skin and membrane irritant and causes chemical burns. In the United States, maximum authorized concentrations are 15 ppm in rinse-offs (of a mixture in the ratio 3:1 of 5-chloro-2-methylisothiazol 3(2H)-one and 2-methylisothiazol-3 (2H)-one).[6] In Canada, methylchloroisothiazolinone may only be used in rinse-off products in combination with methylisothiazolinone, the total concentration of the combination may not exceed 15 ppm.[7]
Methylisothiazolinone is considered safe in the allowed amount in rinse-off products (0.1%) and safe in leave-in products when formulated to be non-sensitizing.[8]
Incidents
An overdose of Kathon by aircraft maintenance personnel, using 38 times the correct amount, resulted in damage to both engines of a Titan Airways aircraft in February 2020. After losing both engines in succession, the Airbus A321 made an emergency landing at London Gatwick Airport.[9] The maintenance procedures specified the Kathon to be diluted to 100 PPM by volume, but with the aircraft maintenance technician being unfamiliar with the term "PPM" and the term not being defined in the aircraft maintenance manuals, the technician instead used an online calculator to convert PPM to percentages, misinterpreted the answer, and added 30 kg of Kathon to each wing tank, which was over 38 times the required amount. Over the course of the next day, the Kathon progressively caused more and more damage to the engines, finally resulting in an emergency landing.[10]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.