Caryophyllene
Chemical compound From Wikipedia, the free encyclopedia
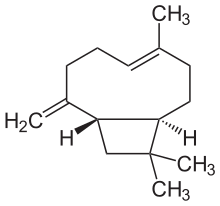
Caryophyllene (/ˌkærioʊˈfɪliːn/), more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that occurs widely in nature. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature. [3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,4E,9S)-4,11,11-Trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | |
| Other names
β-Caryophyllene trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.588 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Density | 0.9052 g/cm3 (17 °C)[1] |
| Boiling point | 262–264 °C (504–507 °F; 535–537 K)[2] |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H304, H317 | |
| P261, P272, P280, P301+P316, P302+P352, P321, P331, P333+P317, P362+P364, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
Caryophyllene can be produced synthetically,[4] but it is invariably obtained from natural sources because it is widespread. It is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops.[3] It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and α-humulene (obsolete name: α-caryophyllene), a ring-opened isomer.
Caryophyllene is one of the chemical compounds that contributes to the aroma of black pepper.[5]
Basic research
β-Caryophyllene is under basic research for its potential action as an agonist of the cannabinoid receptor type 2 (CB2 receptor).[6] In other basic studies, β-caryophyllene has a binding affinity of Ki = 155 nM at the CB2 receptors.[7]
β-Caryophyllene has the highest cannabinoid activity compared to the ring opened isomer α-caryophyllene humulene which may modulate CB2 activity.[8] To compare binding, cannabinol binds to the CB2 receptors as a partial agonist with an affinity of Ki = 126.4 nM,[9] while delta-9-tetrahydrocannabinol binds to the CB2 receptors as a partial agonist with an affinity of Ki = 36 nM.[10]
Safety
Caryophyllene has been given generally recognized as safe (GRAS) designation by the FDA and is approved by the FDA for use as a food additive, typically for flavoring.[11][12] Rats given up to 700 mg/kg daily for 90 days did not produce any significant toxic effects.[13] Caryophyllene has an LD50 of 5,000 mg/kg in mice.[14][15]
Metabolism and derivatives
14-Hydroxycaryophyllene oxide (C15H24O2) was isolated from the urine of rabbits treated with (−)-caryophyllene (C15H24). The X-ray crystal structure of 14-hydroxycaryophyllene (as its acetate derivative) has been reported.[16]
The metabolism of caryophyllene progresses through (−)-caryophyllene oxide (C15H24O) since the latter compound also afforded 14-hydroxycaryophyllene (C15H24O) as a metabolite.[17]
- Caryophyllene (C15H24) → caryophyllene oxide (C15H24O) → 14-hydroxycaryophyllene (C15H24O) → 14-hydroxycaryophyllene oxide (C15H24O2).
Caryophyllene oxide,[18] in which the alkene group of caryophyllene has become an epoxide, is the component responsible for cannabis identification by drug-sniffing dogs[19][20] and is also an approved food additive, often as flavoring.[12] Caryophyllene oxide may have negligible cannabinoid activity.[21]
Natural sources
Summarize
Perspective
The approximate quantity of caryophyllene in the essential oil of each source is given in square brackets ([ ]):
- Cannabis (Cannabis sativa) [3.8–37.5% of cannabis flower essential oil][22]
- Black caraway (Carum nigrum) [7.8%][23]
- Cloves (Syzygium aromaticum) [1.7–19.5% of clove bud essential oil][24]
- Hops (Humulus lupulus)[25] [5.1–14.5%][26]
- Basil (Ocimum spp.)[27] [5.3–10.5% O. gratissimum; 4.0–19.8% O. micranthum][28]
- Oregano (Origanum vulgare)[29] [4.9–15.7%][30][31]
- Black pepper (Piper nigrum) [7.29%][5]
- Lavender (Lavandula angustifolia) [4.62–7.55% of lavender oil][32][33]
- Rosemary (Rosmarinus officinalis)[34] [0.1–8.3%][citation needed]
- True cinnamon (Cinnamomum verum) [6.9–11.1%][35]
- Malabathrum (Cinnamomum tamala) [25.3%][36]
- Ylang-ylang (Cananga odorata) [3.1–10.7%]
- Copaiba oil (Copaifera)[37][38]
Biosynthesis
Caryophyllene is a common sesquiterpene among plant species. It is biosynthesized from the common terpene precursors dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP). First, single units of DMAPP and IPP are reacted via an SN1-type reaction with the loss of pyrophosphate, catalyzed by the enzyme GPPS2, to form geranyl pyrophosphate (GPP). This further reacts with a second unit of IPP, also via an SN1-type reaction catalyzed by the enzyme IspA, to form farnesyl pyrophosphate (FPP). Finally, FPP undergoes QHS1 enzyme-catalyzed intramolecular cyclization to form caryophyllene.[39]
Compendial status
Further reading
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. (2001). "Local anaesthetic activity of beta-caryophyllene". Farmaco. 56 (5–7): 387–389. doi:10.1016/S0014-827X(01)01092-8. hdl:2158/397975. PMID 11482764.
Notes and references
Wikiwand - on
Seamless Wikipedia browsing. On steroids.

