Aluminium iodide
Chemical compound From Wikipedia, the free encyclopedia
Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine[4] or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.[5]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Aluminium iodide | |
| Other names
Aluminium(III) iodide Aluminum iodide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.029.140 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
| UN number | UN 3260 |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| AlI3, AlI3·6H2O (hexahydrate) | |
| Molar mass | 407.695 g/mol (anhydrous) 515.786 g/mol (hexahydrate)[1] |
| Appearance | white (anhydrous) or yellow powder (hexahydrate)[1] |
| Density | 3.98 g/cm3 (anhydrous)[1] 2.63 g/cm3 (hexahydrate)[2] |
| Melting point | 188.28 °C (370.90 °F; 461.43 K) (anhydrous) 185 °C, decomposes (hexahydrate)[1][2] |
| Boiling point | 382 °C (720 °F; 655 K) anhydrous, sublimes[1] |
| very soluble, partial hydrolysis | |
| Solubility in alcohol, ether | soluble (hexahydrate) |
| Structure[3] | |
| Monoclinic, mP16 | |
| P21/c, No. 14 | |
a = 1.1958 nm, b = 0.6128 nm, c = 1.8307 nm α = 90°, β = 90°, γ = 90° | |
Formula units (Z) |
8 |
| Thermochemistry[1] | |
Heat capacity (C) |
98.7 J/(mol·K) |
Std molar entropy (S⦵298) |
195.9 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
−302.9 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
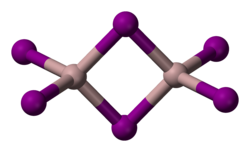
Structure
Solid AlI
3 is dimeric, consisting of Al
2I
6, similar to that of AlBr
3.[3] The structure of monomeric and dimeric forms have been characterized in the gas phase.[6] The monomer, AlI
3, is trigonal planar with a bond length of 2.448(6) Å, and the bridged dimer, Al
2I
6, at 430 K is a similar to Al
2Cl
6 and Al
2Br
6 with Al−I bond lengths of 2.456(6) Å (terminal) and 2.670(8) Å (bridging). The dimer is described as floppy with an equilibrium geometry of D2h.
Aluminium(I) iodide
The name "aluminium iodide" is widely assumed to describe the triiodide or its dimer. In fact, a monoiodide also enjoys a role in the Al–I system, although the compound AlI is unstable at room temperature relative to the triiodide:[7]
- 3 AlI → AlI3 + 2 Al
An illustrative derivative of aluminium monoiodide is the cyclic adduct formed with triethylamine, Al
4I
4(NEt
3)
4.
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
