Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
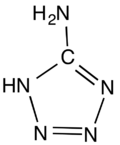
5-Aminotetrazole is an organic compound with the formula HN4CNH2. It is a white solid that can be obtained both in anhydrous and hydrated forms.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H-1,2,3,4-Tetrazol-5-amine | |
| Other names
5-ATZ | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.022.348 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3N5 | |
| Molar mass | 85.070 g·mol−1 |
| Appearance | White solid |
| Density | 1.502 g/cm3 |
| Melting point | 201–205 °C (394–401 °F; 474–478 K) |
| Hazards | |
| GHS labelling: | |
  | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The molecule is planar.[1] The hydrogen bonding pattern in the hydrate supports the assignment of NH being adjacent to carbon in the ring.[2]
A synthesis of 5-aminotetrazole through the action of nitrous acid on aminoguanidine was reported by Johannes Thiele in 1892.[3]
The exact structure of the compound was not known at the time, although it was known to crystallize as a monohydrate. The correct structural formula was published in 1901 by Arthur Hantzsch, who obtained it from the reaction between cyanamide and hydrazoic acid.[4]
To avoid direct handling of the problematic hydrazoic acid, a mixture of sodium azide and hydrochloric acid has been used to give the monohydrate at 73% yield.[5]
In a more efficient and controllable one-pot synthesis, cyanamide is treated with hydrazine hydrochloride to give aminoguanidine hydrochloride, which is then diazotized as in Thiele's original process. Addition of ammonia or sodium hydroxide followed by heat-induced cyclization gives the anhydrous product in 74% yield.[6][7]
The structure of 5-aminotetrazole has been determined several times by X-ray crystallography, both as the anhydrous[8] and monohydrated forms.[9] The structures are very similar, consisting of a planar molecule, including the amino group.
5-Aminotetrazole has found applications in heterocyclic chemistry, particularly as a synthon for some multicomponent reactions.[10]
The N-4 is basic as indicated by its binding to metal halides, such as the coordination complex [CoCl2(aminotetrazole)4.[11]
The compound has a particularly high nitrogen content of 80%. Partly for this reason, the compound is prone to decomposition to nitrogen gas (N2). It has been widely investigated for gas-generating systems, such as airbags and blowing agents.[12]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.