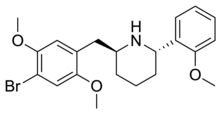
DMBMPP, or 2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine, is a 2-benzylpiperidine analog of the hallucinogenic N-benzylphenethylamine 25B-NBOMe and was discovered in 2011 by Jose Juncosa in the group of David E. Nichols at Purdue University.[1][2] DMBMPP differs from 25B-NBOMe by incorporating the amine within a piperidine ring, making for a more rigid molecular structure than that of the open-chain 25B-NBOMe. The presence of the piperidine ring introduces two stereocenters, thus, four stereoisomers of this compound can be made.
 | |
| Clinical data | |
|---|---|
| Other names | Juncosamine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H26BrNO3 |
| Molar mass | 420.347 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pharmacology
The (S,S)-isomer ((2S,6S)-DMBMPP) is the most selective agonist for the human 5-HT2A receptor yet discovered, with a Ki of 2.5 nM at the human 5-HT2A receptor and with 124-fold selectivity for 5-HT2A over the structurally similar 5-HT2C-receptor.[2] Together with 25CN-NBOH,[3] (2S,6S)-DMBMPP is the only known 5-HT2A agonist to exhibit this level of selectivity.
| Ligand | Ki ± SEM (nM) | Ki ± SEM (nM) | Ki ± SEM (nM) |
|---|---|---|---|
| [3H] ketanserin | [3H] mesulergine | fold selectivity | |
| h5-HT2A | h5-HT2C | h5-HT2C/h5-HT2A | |
| 2C-B | 6.0 ± 0.3 | 23.8 ± 2.6 | 9.5 |
| 25B-NBOMe | 0.19 ± 0.01 | 4.0 ± 0.4 | 21 |
| (±)-DMBMPP | 5.3 ± 0.3 | 520 ± 22 | 98 |
| (S,S)-(−)-DMBMPP | 2.5 ± 0.1 | 310 ± 42 | 124 |
| (R,R)-(+)-DMBMPP | 2,100 ± 171 | 28,600 ± 4700 | 27 |
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
