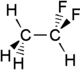
1,1-Difluoroethane
Chemical compound From Wikipedia, the free encyclopedia
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential than other hydrofluorocarbons and a shorter atmospheric lifetime (1.4 years).[2][3]
| |||
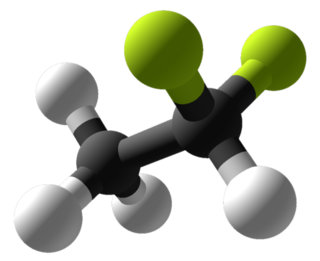 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1-Difluoroethane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.788 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4F2 | |||
| Molar mass | 66.05 g/mol | ||
| Density | 900 g/L @ 25 °C | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | −24.7 °C (−12.5 °F; 248.5 K) | ||
| Critical point (T, P) | 113.45 °C | ||
| 0.54% @ 0 °C | |||
| Vapor pressure |
| ||
| Viscosity | 8.87 μPa·s (0.00887 cP) @ 25 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Extremely flammable | ||
| GHS labelling: | |||
 | |||
| Danger | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | SDS for 1,1-difluoroethane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production
1,1-Difluoroethane is a synthetic substance that is produced by the mercury-catalyzed addition of hydrogen fluoride to acetylene:[4]
- HCCH + 2 HF → CH3CHF2
The intermediate in this process is vinyl fluoride (C2H3F), the monomeric precursor to polyvinyl fluoride.
Uses
With a relatively low global warming potential (GWP) index of 124 and favorable thermophysical properties, 1,1-difluoroethane has been proposed as an environmentally friendly alternative to R134a. Despite its flammability, R152a also presents operating pressures and volumetric cooling capacity (VCC) similar to R134a so it can be used in large chillers[5] or in more particular applications like heat pipe finned heat exchangers.[6]
In addition, 1,1-difluoroethane is also commonly used in gas dusters and numerous other retail aerosol products, particularly those subject to stringent volatile organic compound (VOC) requirements.
The molecular weight of difluoroethane is 66, making it a useful and convenient tool for detecting vacuum leaks in Gas chromatography–mass spectrometry (GC-MS) systems. The cheap and freely available gas has a molecular weight and fragmentation pattern (base peak 51 m/z in typical EI-MS,[7] major peak at 65 m/z) distinct from anything in air. If mass peaks corresponding to 1,1-difluoroethane are observed immediately after spraying a suspect leak point, leaks may be identified.
Safety
Difluoroethane is an extremely flammable gas, which decomposes rapidly on heating or burning, producing toxic and irritating fumes, including hydrogen fluoride and carbon monoxide.[8]
In a DuPont study, rats were exposed to up to 25,000 ppm (67,485 mg/m3) for six hours daily, five days a week for two years. This has become the no-observed-adverse-effect level for this substance. Prolonged exposure to 1,1-difluoroethane has been linked in humans to the development of coronary disease and angina.[9] Repeated or sufficiently high levels of exposure, particularly purposeful inhalation, can precipitate fatal cardiac arrhythmia.[10]
Abuse
Difluoroethane is an intoxicant with abuse potential.[10][11][12][13] It appears to act primarily through GABAA and glutamate receptors.[14][15] Fatalities linked to difluoroethane abuse include actress Skye McCole Bartusiak, singer Aaron Carter and wrestler Mike Bell.[16] Bitterants, added voluntarily to some brands to deter purposeful inhalation, are often not legally required; they do not negate or counteract difluoroethane's intoxicating effects.
Environmental abundance


Most production, use, and emissions of HFC-152a have occurred within Earth's more industrialized and populated northern hemisphere following the substance's introduction in the 1990s. Its concentration in the northern troposphere reached an annual average of about 10 parts per trillion by year 2011.[17] The concentration of HFC-152a in the southern troposphere is about 50% lower due to its removal rate (i.e. lifetime) of about 1.5 years being similar in magnitude to the global atmospheric mixing time of one to two years.[18]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.