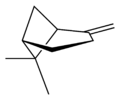
Β-Pinene
Chemical compound From Wikipedia, the free encyclopedia
β-Pinene is a monoterpene, an organic compound found in plants. It is the less abundant of the two isomers of pinene, the other being α-pinene.[3] It is a colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.
 | |||
| |||
| Names | |||
|---|---|---|---|
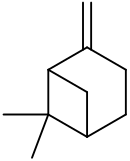
| IUPAC names
6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane Pin-2(10)-ene | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane 2(10)-Pinene Nopinene Pseudopinene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.004.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.872 g/mL | ||
| Melting point | −61.54 °C; −78.77 °F; 211.61 K[1] | ||
| Boiling point | 165–167 °C; 329–332 °F; 438–440 K[2] | ||
| Thermochemistry | |||
Std enthalpy of combustion (ΔcH⦵298) |
−6214.1±2.9 kJ/mol[1] | ||
| Hazards | |||
| GHS labelling: | |||
    | |||
| Danger | |||
| H226, H304, H315, H317, H410 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P272, P273, P280, P301+P310, P302+P352, P303+P361+P353, P321, P331, P332+P313, P333+P313, P362, P363, P370+P378, P391, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 36 °C (97 °F; 309 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
β-Pinene is one of the most abundant compounds released by forest trees.[4] If oxidized in air, the allylic products of the pinocarveol and myrtenol family prevail.[5]
Sources
Many plants from many botanical families contain the compound, including:
- Cuminum cyminum[6][7]
- Humulus lupulus[8]
- Pinus pinaster[5]
- Clausena anisata
- Cannabis sativa[9]
- Piper nigrum[10]
- Myristica fragans .[10]
- Citrus aurantiifolia[10]
- Pistacia lentiscus[10]
The clear compound is produced by distillation of turpentine oils.[11]
Uses
β-Pinene is usedin the production of other aroma compounds. It converts to myrcene upon heating at 500 °C. Nerol is obtained by careful fractional distillation of crude nerol from myrcene[12]).[13]
Reaction with formaldehyde (Prins reaction) converts β-pinene to nopol. When nopol is acetylated, the result is nopyl acetate, which is used as fragrance material.[11][14]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.