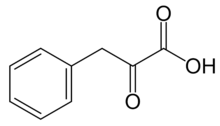
Phenylpyruvic acid is the organic compound with the formula C6H5CH2C(O)CO2H. It is a keto acid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Oxo-3-phenylpropanoic acid | |
| Other names
Phenylpyruvate; 3-Phenylpyruvic acid; Keto-phenylpyruvate; beta-Phenylpyruvic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.317 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.160 g·mol−1 |
| Melting point | 155 °C (311 °F; 428 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Occurrence and properties
The compound exists in equilibrium with its (E)- and (Z)-enol tautomers.[citation needed] It is a product from the oxidative deamination of phenylalanine.
When the activity of the enzyme phenylalanine hydroxylase is reduced, the amino acid phenylalanine accumulates and gets converted into phenylpyruvic acid (phenylpyruvate), which leads to 'Phenylketonuria (PKU)' instead of 'tyrosine' which is the normal product of phenylalanine hydroxylase.
Preparation and reactions
It can be prepared by many methods. Classically it is produced from aminocinnamic acid derivatives.[1] It has been prepared by condensation of benzaldehyde and glycine derivatives to give phenylazlactone, which is then hydrolyzed with acid- or base-catalysis.[2] It can also be synthesized from benzyl chloride by double carbonylation.[3][4]
Reductive amination of phenylpyruvic acid gives phenylalanine.
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
