In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is R1R2C=N+(−O−)(−R3), where R3 is not a hydrogen. Their primary application is intermediates in chemical synthesis. A nitrone is a 1,3-dipole used in cycloadditions, and a carbonyl mimic.

Structure
Nitrones, as a tetrasubstituted double bond, admit cis–trans isomerism.[1]: 474
Generation of nitrones
Typical nitrone sources are hydroxylamine oxidation or condensation with carbonyl compounds. Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate.[1]: 476 [2]: 332–333 The most general reagent used for the oxidation of hydroxylamines is aqueous mercuric oxide:[1]: 476 [3]

However, a hydroxylamine with two α hydrogens may unsaturate on either side. Carbonyl condensation avoids this ambiguity...[4]

...but is inhibited if both ketone substituents are bulky.[1]: 477
In principle, N-alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack.[1]: 479 [2]: 334
Reactions
Some nitrones oligomerize:[1]: 483 [2]: 334,337-338 [5]
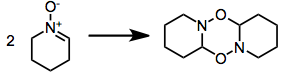
Syntheses with nitrone precursors obviate the issue with increased temperature, to exaggerate entropic factors; or with a nitrone excess.
Carbonyl mimic
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction. The α carbon is an electrophile and the β carbon a nucleophile; that is, nitrones polarize like carbonyls and nitriles but unlike nitro compounds and vinyl sulfur derivatives.[1]: 483 [2]: 338–340
Nitrones hydrolyze extremely easily to the corresponding carbonyl and N-hydroxylamine.[1]: 491 [2]: 344
1,3-dipolar cycloadditions
As 1,3‑dipoles, nitrones perform [3+2] cycloadditions.[6] For example, a dipolarophilic alkene combines to form isoxazolidine:

Other ring-closing reactions are known,[7] including formal [3+3] and [5+2] cycloadditions.[6]
Isomerization
Deoxygenating reagents, light, or heat all catalyze rearrangement to the amide. Acids catalyze rearrangement to the oxime ether.[1]: 489–490 [2]: 345–347
Reduction
Hydrides add to give hydroxylamines. Reducing Lewis acids (e.g. metals, SO2) deoxygenate to the imine instead.[1]: 490 [2]: 343
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
