Loading AI tools
Amino acid From Wikipedia, the free encyclopedia
Threonine (symbol Thr or T)[2] is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli.[3] It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG).
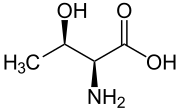 Skeletal formula of L-threonine | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Threonine | |||
| Other names
2-Amino-3-hydroxybutanoic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.704 | ||
| EC Number |
| ||
| |||
| KEGG |
| ||
PubChem CID |
| ||
| UNII |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H9NO3 | |||
| Molar mass | 119.120 g·mol−1 | ||
| (H2O, g/dl) 10.6(30°),14.1(52°),19.0(61°) | |||
| Acidity (pKa) | 2.63 (carboxyl), 10.43 (amino)[1] | ||
| Supplementary data page | |||
| Threonine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine. Phosphothreonine has three potential coordination sites (carboxyl, amine and phosphate group) and determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is important to explain the function of the phosphothreonine in biological processes.[4]
Threonine was the last of the 20 common proteinogenic amino acids to be discovered. It was discovered in 1935 by William Cumming Rose,[5] collaborating with Curtis Meyer. The amino acid was named threonine because it was similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C4H8O5[6]
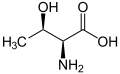  |
| L-threonine (2S,3R) and D-threonine (2R,3S) |
 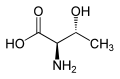 |
| L-allothreonine (2S,3S) and D-allothreonine (2R,3R) |
Threonine is one of two proteinogenic amino acids with two stereogenic centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single stereoisomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The stereoisomer (2S,3S), which is rarely present in nature, is called L-allothreonine.[7]
As an essential amino acid, threonine is not synthesized in humans, and needs to be present in proteins in the diet. Adult humans require about 20 mg/kg body weight/day.[8] In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[9] Enzymes involved in a typical biosynthesis of threonine include:

Threonine is metabolized in at least three ways:
The degradation of threonine is impaired in the following metabolic diseases:
Effects of threonine dietary supplementation have been researched in broilers.[17]
An essential amino acid, threonine is involved in the metabolism of fats, the creation of proteins, the proliferation and differentiation of embryonic stem cells, and the health and function of the intestines. Animal health and illness are strongly correlated with the need for and metabolism of threonine. Intestinal inflammation and energy metabolism disorders in animals may be alleviated by appropriate amounts of dietary threonine. Nevertheless, because these effects pertain to the control of nutrition metabolism, more research is required to confirm the results in various animal models. Furthermore, more research is needed to understand how threonine controls the dynamic equilibrium of the intestinal barrier function, immunological response and gut flora.[18]
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, black turtle bean[19] and sesame seeds.[20]
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[21]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.