Top Qs
Timeline
Chat
Perspective
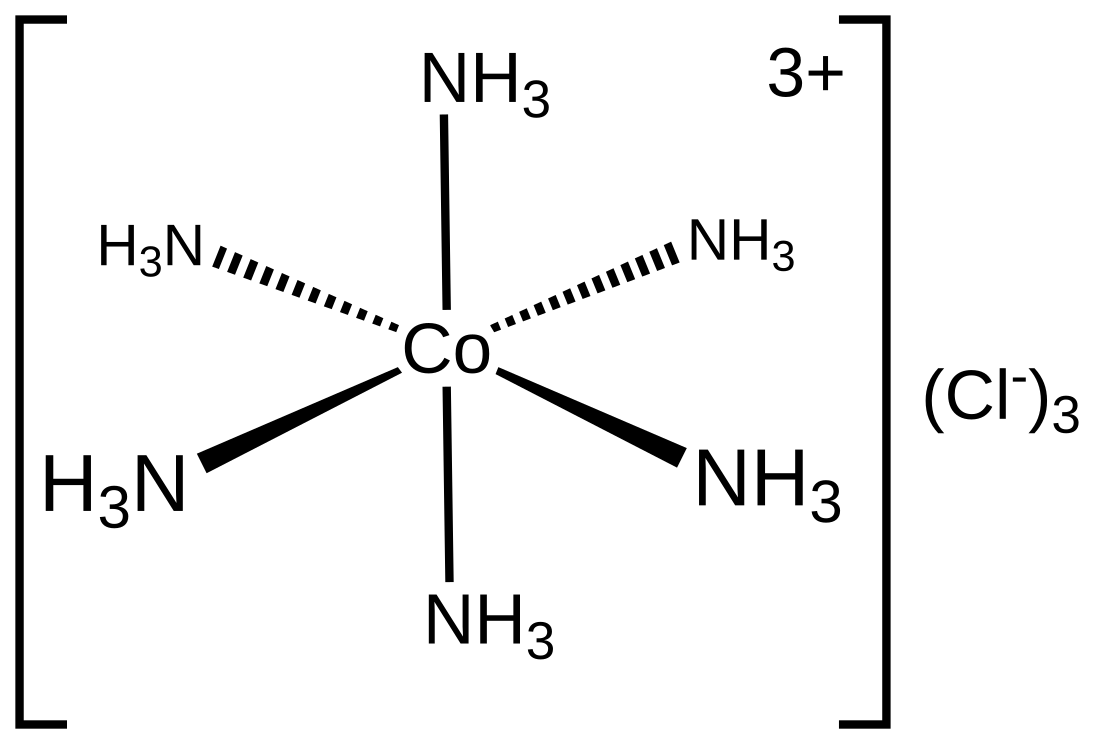
Hexaamminecobalt(III) chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.
Remove ads
Properties and structure
[Co(NH3)6]3+ is diamagnetic, with a low-spin 3d6 octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal complex. As a manifestation of its inertness, [Co(NH3)6]Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation.[1] In contrast, labile metal ammine complexes, such as [Ni(NH3)6]Cl2, react rapidly with acids, reflecting the lability of the Ni(II)–NH3 bonds. Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chloride ions in [Co(NH3)6]Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, iodide, sulfamate to afford the corresponding [Co(NH3)6]X3 derivative. Such salts are orange or bright yellow and display varying degrees of water solubility. The chloride ion can be also exchanged with more complex anions such as the hexathiocyanatochromate(III), yielding a pink compound with formula [Co(NH3)6] [Cr(SCN)6], or the ferricyanide ion.[citation needed]
Remove ads
Preparation
[Co(NH3)6]Cl3 is prepared by treating cobalt(II) chloride with ammonia and ammonium chloride followed by oxidation. Oxidants include hydrogen peroxide or oxygen in the presence of charcoal catalyst.[1] This salt appears to have been first reported by Fremy.[2]
The acetate salt can be prepared by aerobic oxidation of cobalt(II) acetate, ammonium acetate, and ammonia in methanol.[3] The acetate salt is highly water-soluble to the level of 1.9 M (20 °C), versus 0.26 M for the trichloride.
Remove ads
Uses in the laboratory
[Co(NH3)6]3+ is a component of some structural biology methods (especially for DNA or RNA, where positive ions stabilize tertiary structure of the phosphate backbone), to help solve their structures by X-ray crystallography[4] or by nuclear magnetic resonance.[5] In the biological system, the counterions would more probably be Mg2+, but the heavy atoms of cobalt (or sometimes iridium, as in PDB: 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.[6]
[Co(NH3)6]3+ is used to investigate DNA. The cation induces the transition of DNA structure from the classical B-form to the Z-form.[7]
Related compounds
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads