I kemi er en funktionel gruppe den del af et molekyle som bestemmer molekylets kemiske egenskaber og dermed hvilke reaktioner molekylet kan deltage i. Et molekyle kan indeholde mere end en funktionel gruppe, eller slet ingen.
| Klasse | Gruppe | Formel | Strukturformel | Præfiks | Suffiks | Eksempel |
|---|---|---|---|---|---|---|
| Acylhalid (syreklorid) | Haloformyl | RCOX |  |
haloformyl- | -oylhalid | Acetylklorid (Ethanoylklorid) |
| Alkohol | Hydroxyl | ROH | hydroxy- | -ol | 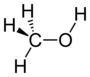 Methanol | |
| Aldehyd | Aldehyd | RCHO |  |
oxo- | -al | 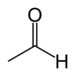 Acetaldehyd (Ethanal) |
| Alkan | Alkyl | RH | alkyl- | -an | 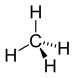 Methan | |
| Alken | Alkenyl | R2C=CR2 | 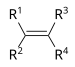 |
alkenyl- | -en |  Ethylen (Ethen) |
| Alkyn | Alkynyl | RC≡CR' | alkynyl- | -yn | Acetylen (Ethyn) | |
| Amid | Carboxamid | RCONR2 |  |
carboxamido- | -amid |  Acetamid (Ethanamid) |
| Aminer | Primær amin | RNH2 | amino- | -amin | 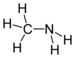 Metylamin (Methanamin) | |
| Sekundær amin | R2NH |  |
amino- | -amin | Dimethylamin | |
| Tertiær amin | R3N | 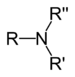 |
amino- | -amin | 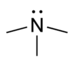 Trimethylamin | |
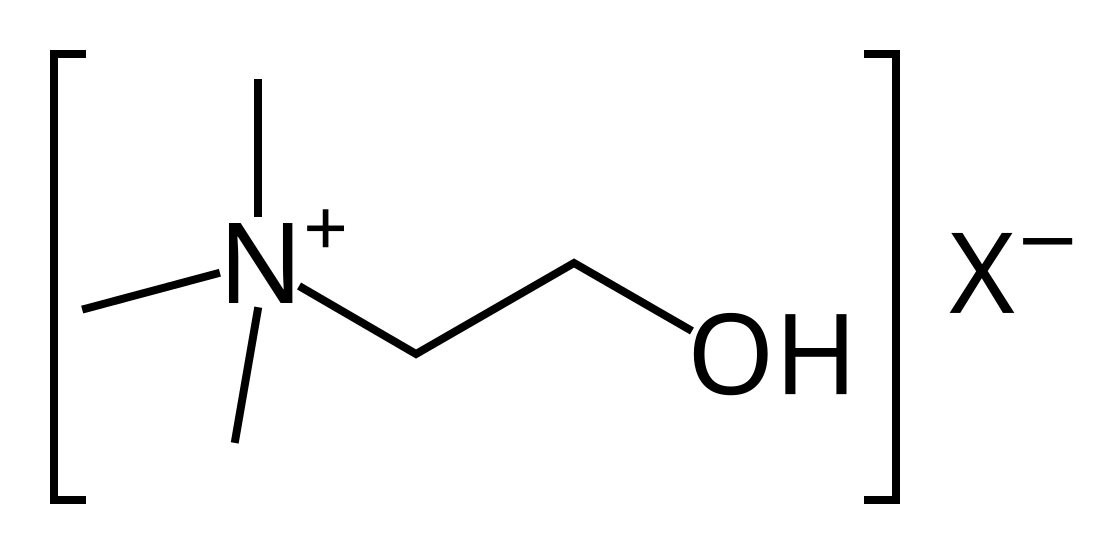
| Kvarternær ammoniumkation | R4N+ | 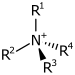 |
ammonio- | -ammonium |  Kolin | |
| Azoforbindelse | Azo (Diimid) |
RN2R' | azo- | -diazen | 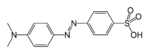 Methylorange (p-dimethylamino- azobenzensulfonsyre) | |
| Toluenderivater | Benzyl | RCH2C6H5 RBn |
benzyl- | 1-(substituent)toluen | Benzylbromid (1-Bromtoluen) | |
| Carbonat | Carbonatester | ROCOOR | alkyl carbonat | |||
| Carboxylat | Carboxylat | RCOO− | 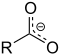 |
carboxy- | -oat | Natriumacetat (Natriumethanoat) |
| Carboxylsyre | Carboxyl | RCOOH | 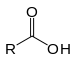 |
carboxy- | -(carboxyl)syre | 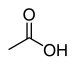 Eddikesyre (Ethansyre) |
| Cyanater | Cyanat | ROCN | cyanato- | alkyl cyanat | ||
| Thiocyanat | RSCN | thiocyanato- | alkyl thiocyanat | |||
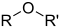
| Æter | Æter | ROR' | alkoxy- | alkyl-alkyl-æter | Diætylæter (Ethoxyethan) | |
| Ester | Ester | RCOOR' | alkyl-alkanoate | Ætylbutyrat (Ætylbutanoat) | ||
| Haloalkan | Halo | RX |  |
halo- | alkylhalid | Chlorethan (Ætylklorid) |
| Hydroperoxid | Hydroperoxy | ROOH | hydroperoxy- | alkylhydroperoxid | Methylethylketonperoxid | |
| Imin | Primær ketimin | RC(=NH)R' |  |
imino- | -imin | |
| Sekundær ketimin | RC(=NR)R' | 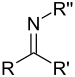 |
imino- | -imin | ||
| Primær aldimin | RC(=NH)H |  |
imino- | -imin | ||
| Sekundær aldimin | RC(=NR')H | 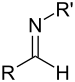 |
imino- | -imin | ||
| Isocyanid | Isocyanid | RNC | isocyano- | alkylisocyanid | ||
| Isocyanater | Isocyanat | RNCO | isocyanato- | alkylisocyanat | Methylisocyanat | |
| Isothiocyanat | RNCS | isothiocyanato- | alkylisothiocyanat | Allylisothiocyanat | ||
| Keton | Carbonyl | RCOR' | 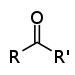 |
keto-, oxo- | -on | 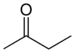 Methylethylketon (Butanon) |
| Nitrat | Nitrat | RONO2 | nitrooxy-, nitroxy- |
alkylnitrat |
 Amylnitrat (1-nitrooxypentan) | |
| Nitril | Nitril | RCN | cyano- |
alkannitril |
 Benzonitril (Phenylcyanid) | |
| Nitrit | Nitrosooxy | RONO | nitrosooxy- |
alkylnitrit |
 Amylnitrit (3-methyl-1-nitrosooxybutan) | |
| Nitroforbindelse | Nitro | RNO2 | 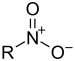 |
nitro- | 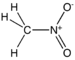 Nitromethan | |
| Nitrosoforbindelse | Nitroso | RNO | nitroso- | Nitrosobenzen | ||
| Peroxid | Peroxy | ROOR | peroxy- | alkylperoxid | Di-tert-butylperoxid | |
| Benzenderivater | Phenyl | RC6H5 | phenyl- | -benzen | 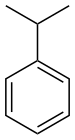 Cumene (2-phenylpropan) | |
| Fosfin | Fosfino | R3P | phosphino- | -phosphan | Methylpropylphosphan | |
| Fosfodiester | Fosfat | HOPO(OR)2 | fosforsyre- di(substituent)ester |
di(substituent)- hydrogenfosfat |
DNA | |
| Phosphosyre | Phosphono | RP(=O)(OH)2 | 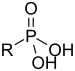 |
phosphono- | substituent phosphonsyre | Benzylphosphonic acid |
| Fosfat | Fosfat | ROP(=O)(OH)2 |  |
phospho- | Glyceraldehyd-3-fosfat | |
| Pyridinderivater | Pyridyl | RC5H4N |
4-pyridyl |
-pyridin |  Nicotin | |
| Sulfid | RSR' | di(substituent)sulfid | Dimethylsulfid | |||
| Sulfon | Sulfonyl | RSO2R' |  |
sulfonyl- | di(substituent)- sulfon |
Dimethylsulfon (Metylsulfonylmetan) |
| Sulfonsyre | Sulfo | RSO3H |  |
sulfo- | substituent- sulfonsyre |
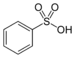 Benzensulfonsyre |
| Sulfoxid | Sulfinyl | RSOR' | 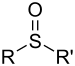 |
sulfinyl- | di(substituent)- sulfoxid |
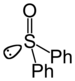 Difenylsulfoxid |
| Thiol | Sulfhydryl | RSH | mercapto-, sulfanyl- | -thiol | Ethanthiol (Ethylmercaptan) |
| Spire Denne artikel om kemi er en spire som bør udbygges. Du er velkommen til at hjælpe Wikipedia ved at udvide den. |
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.